Abstract
Multiple neuron ensemble recordings were obtained simultaneously from both the primary somatosensory (SI) cortex and the ventroposterior medial thalamus (VPM) before and during the combined administration of reversible inactivation of the SI cortex and a reversible subcutaneous block of peripheral trigeminal nerve fibers. This procedure was performed to quantify the contribution of descending corticofugal projections on (i) the normal organization of thalamic somatosensory receptive fields and (ii) the thalamic somatosensory plastic reorganization that immediately follows a peripheral deafferentation. Reversible inactivation of SI cortex resulted in immediate changes in receptive field properties throughout the VPM. Cortical inactivation also significantly reduced but did not completely eliminate the occurrence of VPM receptive field reorganization resulting from the reversible peripheral deafferentation. This result suggests that the thalamic plasticity that is seen immediately after a peripheral deafferentation is dependent upon both descending corticofugal projections and ascending trigeminothalamic projections.
Recent studies demonstrate that the cortical sensory reorganization (1) that immediately follows a peripheral deafferentation (2) is also paralleled by simultaneous plasticity at thalamic and other subcortical levels (3, 4). Further, both the spatial and temporal characteristics of novel cortical and thalamic sensory responses are remarkably similar (4). Based on these results, we proposed that immediate and concurrent cortical and subcortical plasticity results from changes in a dynamic equilibrium between both ascending lemniscal and paralemniscal pathways and descending corticofugal projections that converge at all central processing levels of the somatosensory system (3, 5).
Herein, we began to test this hypothesis by investigating how reversible inactivation of corticofugal projections affected the immediate thalamic reorganization observed in the rat trigeminal somatosensory system after a reversible peripheral sensory deafferentation induced by subcutaneous injection of lidocaine. We observed that cortical inactivation during this peripheral sensory deafferentation significantly reduced both the percentage of thalamic ventral posterior medial (VPM) neurons exhibiting new unmasked sensory responses and the size of these unmasked responses but did not abolish the occurrence of thalamic reorganization. As described below, these results support our hypothesis by demonstrating that thalamic plasticity is defined by contributions from both ascending trigeminothalamic pathways and descending corticothalamic projections.
MATERIALS AND METHODS
The procedures for surgical electrode implants, single unit discrimination, whisker stimulation, and statistical analyses have been described in detail elsewhere (4, 6, 7). Briefly, microwire electrode arrays were chronically implanted in both the infragranular layer of the barrel region of primary somatosensory (SI) cortex (8 microwires per array) and the VPM thalamus (16 microwires per array) in 12 female Long–Evans rats (250–300 g at surgery; surgical anesthesia, pentobarbital at 50 mg/kg, i.p.). In addition, a stainless steel infusion guide cannula was also implanted in SI cortex. The tip of the cannula was positioned in the infragranular layer, 400 μm caudal to the SI microwire array (see Fig. 1A). All rats received 7 days of post-surgical recovery.
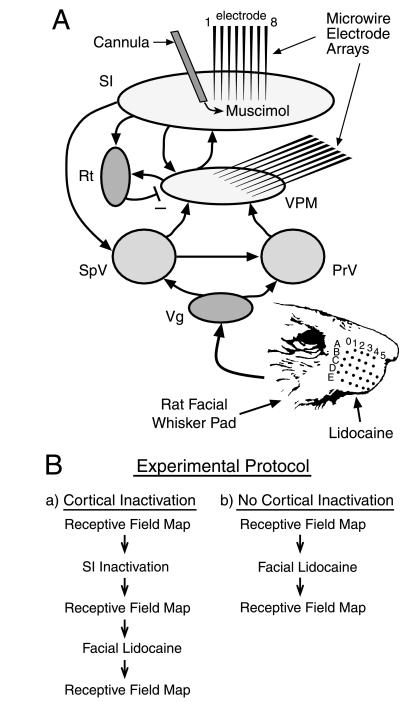
Figure 1.
(A) Simplified schematic diagram of the rat trigeminal somatosensory system. Both feed-forward (from the periphery to cortex) and feed-back (from cortex to subcortical nuclei) projections are illustrated to highlight the recurrent nature of this neural network. Also shown is the characteristic pattern of rows (rows A–E) and columns (columns 0–5) of the rat’s facial whiskers. (B) Schematic diagram of the experimental protocol. PrV, principal trigeminal nucleus; Rt, reticular thalamic nucleus; SI, primary somatosensory cortex; SpV, spinal trigeminal nucleus; Vg, trigeminal ganglion; VPM, ventroposterior medial thalamic nucleus.
After recovery, rats were lightly anesthetized (pentobarbital) and single-unit neuronal activity was isolated on the microwire electrodes (up to four single units per wire) in the SI cortex and VPM. After single-unit isolation, 16–25 facial whiskers were individually stimulated (300 stimulations per whisker) with a computer-controlled probe (whisker deflection, 3–5 degrees; stimulus duration, 100 ms; stimulus rate, 1 Hz). Receptive field (RF) properties of each of the isolated neurons were defined by quantitatively analyzing the recorded neuronal activity after whisker stimulation.
Immediately after completion of the whisker stimulation, the γ-aminobutyric acid (GABA) agonist muscimol (150 ng in 150 nl of saline) was infused into the SI cortex through a 33-gauge infusion cannula lowered through the chronically implanted guide cannula (see Fig. 1A). This dose of muscimol was chosen to inactivate the barrel region of SI cortex for several hours (see Fig. 2). While SI remained inactive, the same set of whiskers (as above) was again stimulated (see flow chart, Fig. 1Ba). The resulting RF properties for each neuron while the SI cortex was inactive were compared with the previous RF properties recorded with SI intact. The use of the word intact (here and below) refers to a functionally intact SI cortex, in other words, fully active. Finally, while SI remained inactive, lidocaine (∼20 μl, 1%) was subcutaneously injected into the face just adjacent to the whisker pad to induce a temporary peripheral deafferentation of a small subset of facial whiskers. The same whiskers (as above) were again stimulated to characterize the lidocaine-induced immediate reorganization of RFs in VPM while the SI cortex remained inactive. In other experiments, only facial lidocaine was administered; the SI cortex was not inactivated (see flow chart, Fig. 1Bb). Reorganization of VPM RFs after facial lidocaine was compared with and without cortical inactivation (4).
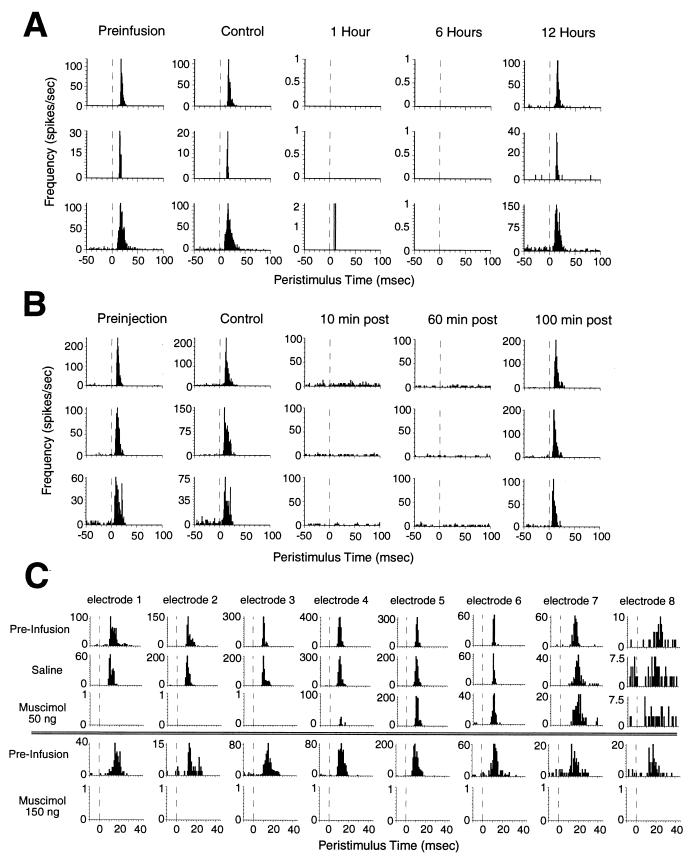
Figure 2.
(A) Examples of the duration and reversibility of muscimol inactivation. Peristimulus time histograms (PSTHs) for three SI neurons resulting from repetitively stimulating a single whisker before (preinfusion), after infusion of saline vehicle (150 nl) into the SI cortex (control), and 1, 6, and 12 h after infusion of muscimol (150 ng) into the SI cortex. Although infusion of saline vehicle had no effect on cortical activity, muscimol completely abolished cortical activity for approximately 9 h. The inactivation was completely reversible: responses returned to baseline within 12 h. (B) Examples of the duration and reversibility of lidocaine-induced peripheral deafferentation. PSTHs for three VPM neurons before any lidocaine injections (preinjection), after a control injection, and 10, 60, and 100 min after a subcutaneous injection (20 μl, 1%) near the stimulated whisker. The effective lidocaine injection completely anesthetized the stimulated region of the face for at least 60 min, and the effect was completely reversible. (C) Spatial extent of muscimol inactivation of the SI cortex. Each of the eight columns represents the PSTH from a single neuron isolated on each of the eight microwires of an SI cortical array after stimulation of a single whisker. The muscimol infusion cannula is 250 μm away from microelectrode 1 (microwires extended linearly away from the cannula, each wire 250 μm away from the adjacent wire, see Fig. 1A). Infusion of saline had no effect on neural activity on any of the wires. A low dose of muscimol (50 ng in 50 nl of saline) abolished activity on three or four wires (approximately 1.2 mm). In a different experiment (Lower), a higher dose of muscimol (150 ng in 150 nl of saline) abolished neural activity on all microwires (approximately 2.2 mm). This dose (150 ng) effectively abolished activity throughout the barrel region of the SI cortex and was used in all experiments involving facial lidocaine. The functional spread of each muscimol infusion during each experiment was confirmed by monitoring activity on the cortical array.
After all experiments, animals were injected with a lethal dose of pentobarbital and then perfused with saline and 10% formalin. Small electrolytic marking lesions were made through several of the electrodes (70 μA for 7 s). The brains were removed, fixed in formalin/sucrose, sectioned, and stained with cresyl violet and Prussian blue. Placement of microwires was histologically confirmed. Only neurons recorded on electrodes within the appropriate structures were included in the analyses.
RESULTS
Fig. 1 depicts a summary of the neural structures and connectivity that defines the rat trigeminal somatosensory system and the experimental paradigm used in this study. Both feed-forward (from the periphery to cortex) and feed-back (from cortex to subcortical nuclei) projections are illustrated to highlight the recurrent nature of this neural network. Fig. 2 shows control data that demonstrate the effectiveness of the muscimol-induced cortical block and the lidocaine-induced peripheral deafferentation. By combining simultaneous thalamocortical ensemble recordings with intracortical infusion of muscimol, we were able to accurately quantify and continuously monitor the temporal duration (9–12 h, Fig. 2A) and spatial spread (Fig. 2C) of the reversible cortical inactivation. These ensemble recordings also demonstrated that cortical inactivation could be maintained far longer than the duration (up to 2–4 h) of the reversible peripheral deafferentation produced by the subcutaneous injection of lidocaine (3, 4) (Fig. 2, compare A and B). This guaranteed that corticofugal pathways, including corticothalamic projections, were blocked during and beyond the period of immediate reorganization induced in the VPM nucleus by the peripheral lidocaine block.
The first step in this study was to characterize the effects of cortical inactivation on the organization of thalamic RFs in the rat VPM. These effects were investigated in 231 VPM neurons recorded in 12 adult Long–Evans rats. Because we recorded cortical neuronal activity throughout the process of cortical inactivation, we were able to verify that the dose of muscimol used (150 ng) effectively abolished neural activity throughout the barrel region of the SI cortex (see Fig. 2).
Immediately after the cortical inactivation, 70% of VPM neurons exhibited changes in their RFs. In 52% of these neurons, long-latency components of the RFs (6) were reduced or completely blocked. About 38% of the neurons exhibited unmasking of sensory responses to single whisker stimulation. Finally, 26% of the VPM neurons displayed changes in short-latency responses and 16% exhibited significant temporal shifts from short to long latencies in the sensory responses to single whisker stimulation. Overall, inactivation of the SI cortex resulted in modification of RF size (increases or reductions) which averaged 1.2 ± 0.11 whiskers per VPM neuron (see Fig. 3 for examples). In many instances, the effects described above included both unmasking of novel responses and elimination of existing responses for the same neuron. These results ruled out the possibility that cortical feedback was simply acting as a global tonic modulator of the sensitivity profile or excitability of VPM neurons. Moreover, there was no significant difference between the spontaneous background firing rate of VPM neurons before and after muscimol infusion [t(460) = 1.5; not significant].
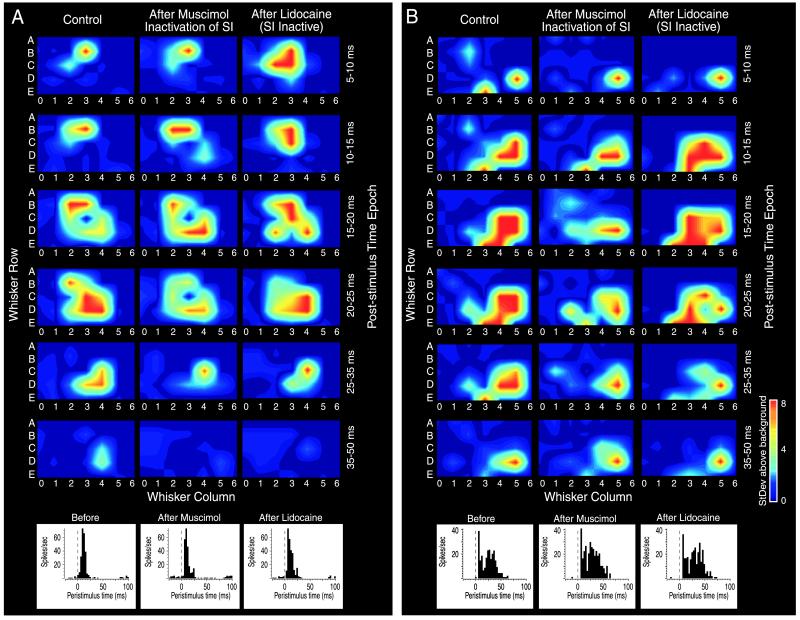
Figure 3.
(A and B) Two examples of individual VPM neurons’ RFs before inactivation of the SI cortex (Left), after inactivation of the SI cortex (Middle), and again after injection of facial lidocaine during cortical inactivation (Right). (Upper) Each column depicts the spatiotemporal properties of the RF by plotting the neuron’s response to stimulation of each of the facial whiskers during six different time epochs (5–10 ms to 35–50 ms) after stimulus onset. In these examples, cortical inactivation resulted in a reduced receptive field size primarily through elimination of longer latency responses. Facial lidocaine injection resulted in unmasking of novel responses. The color code represents the relative variation in response magnitude as follows: dark blue, baseline firing rate; dark red, >8 standard deviations (StDev) above baseline. (Lower) The perievent time histogram resulting from stimulation of the PW during each of the three experimental conditions. In these examples, the responses to PW stimulation were unaffected by muscimol or lidocaine administration, indicating that the receptive field changes observed in each example were not a result of generalized state changes within the animal.
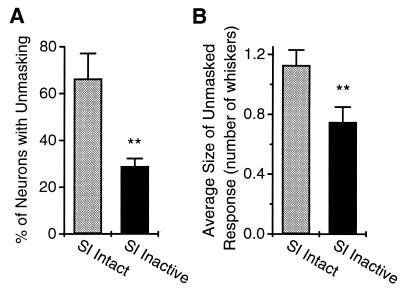
Because the changes in VPM RFs described above remained stable for several hours after cortical inactivation, the next step was to induce a reversible peripheral deafferentation with a subcutaneous lidocaine injection while cortex remained inactive. The resulting lidocaine-induced immediate thalamic reorganization was then quantified and compared with the VPM reorganization seen when lidocaine was administered without cortical inactivation (4) (see Fig. 1B for details of experimental paradigm). Fig. 4 summarizes these results, which demonstrate that cortical inactivation reduced the degree of thalamic reorganization by approximately 50%. This reduction translated into a significantly lower percentage of VPM neurons exhibiting new unmasked sensory responses (from 66 ± 10.7% to 29 ± 3.2%; t(7) = −4.5, P < 0.003; Fig. 4A) and a significantly smaller size of the unmasked sensory responses per neuron after the peripheral deafferentation (from 1.13 ± 0.13 to 0.75 ± 0.11 whiskers, t(11) = 5.9, P < 0.005; Fig. 4B). This decrease in the size of the unmasked response may also be expressed in integer values corresponding to actual numbers of whiskers. With the SI cortex intact, the percentage of VPM neurons in which the size of the unmasked response was zero whiskers was 34%, one whisker was 41%, two were 14%, three were 4%, four were 4%, five were 2%, and six were 1%. In contrast, with the SI cortex inactive, the percentage of VPM neurons in which the size of the unmasked response was zero whiskers was 71%, one was 8%, two were 8%, three were 7%, four were 5%, five were 1%, and six were 0%. The overall extent of the VPM nucleus that underwent reorganization (measured by counting the total number of whiskers that induced unmasked responses in the VPM neural ensemble) did not differ significantly with or without cortical inactivation: 4.0 ± 0.6 vs. 6.1 ± 1.6 whiskers (Fig. 5). Also, the mean latencies of unmasked responses in VPM (with and without cortical inactivation) did not differ significantly (13.74 ± 1.05 ms vs. 13.10 ± 0.6 ms), even though, in both cases, they were much longer than normal minimal response latencies seen in VPM (6).
Figure 4.
(A) Percentage of VPM neurons (mean ± SEM) in the total recorded population showing unmasked responses to whisker stimulation after peripheral lidocaine deafferentation. Cortical inactivation resulted in a significant decrease in the percentage of VPM neurons showing unmasked responses after peripheral deafferentation (from 66 ± 10.7% to 29 ± 3.2%; t(7) = −4.5, P < 0.003). Bars: SI Intact, percentage of VPM neurons showing unmasked responses after facial lidocaine injections in which SI cortex was not inactivated; SI Inactive, percentage of VPM neurons showing unmasked responses after facial lidocaine injections while the SI cortex was inactivated with muscimol. (B) Average change in receptive field size (mean number of whiskers ± SEM) after facial lidocaine for each of the conditions described above. Cortical inactivation resulted in a significant decrease in the size of the unmasked RF after peripheral deafferentation (from 1.13 ± 0.11 to 0.75 ± 0.11 whiskers, t(11)=5.9, P < 0.005).
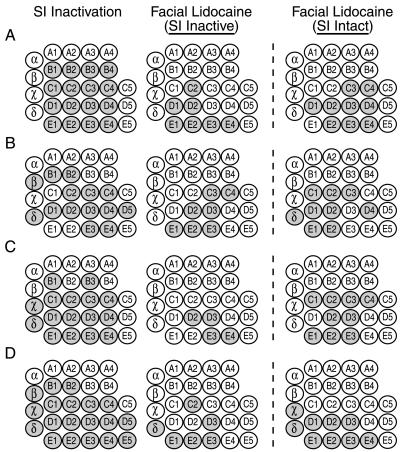
Figure 5.
(A–D) Four examples (from four animals) of the spatial extent of the sensory reorganization process in the VPM after the following manipulations: SI Inactivation, Facial Lidocaine with SI Inactive, and Facial Lidocaine without SI Inactivation. The spatial extent of the reorganization was quantified by measuring the number of stimulated whiskers (shaded circles) that produced novel responses. SI inactivation resulted in reorganization throughout most of the VPM. In contrast, the spatial extent of reorganization after facial lidocaine with SI inactive was much more localized, bordering the site of injection and extending only one or two whisker rows from the injection site. The spatial extent of reorganization after facial lidocaine without SI inactivation (SI Intact) did not differ significantly from the reorganization with SI inactivation. However, there was an overall trend toward a greater spread of reorganization when SI was not inactivated (SI Intact).
DISCUSSION
Overall, the results described in this paper demonstrate that corticofugal pathways are involved in the process of subcortical reorganization, particularly at the thalamic level. They also indicate that feed-forward trigeminothalamic projections contribute to the process of immediate thalamic plasticity. This conclusion is supported by the observation that a significant number of unmasked sensory responses could still be detected after lidocaine injection while the SI cortex remained inactive (Figs. 3 and 5). As the examples in Fig. 3 indicate, the lidocaine-induced thalamic reorganization observed during cortical inactivation still followed the same general patterns observed in animals whose cortex was not inactivated. (i) In neurons whose RFs included at least part of the anesthetized zone, the unmasked RFs tended to be located away from the anesthetized region. (ii) If the anesthetized region did not include the principal whisker (PW) of a given VPM neuron, the unmasking occurred primarily in the far surround of the neuron’s RF. (iii) The immediate reorganization within the VPM during cortical inactivation also included increases (21% of the neurons) and reductions (8%) in response latency and variations in maximum response magnitude (RM; 19% of neurons increased RM and 17% decreased RM).
Lidocaine injections into different regions of the whisker pad did not appear to have any differential effects on unmasking of VPM RFs. We also did not observe any systematic differences in the patterns of changes in VPM RFs relative to the position of specific lidocaine injection sites and the whiskers inactivated. Finally, of the entire population of recorded VPM neurons, 63 (27%) had RFs located far from the region of lidocaine inactivation. There were no significant changes observed in the RFs of these neurons.
It is well known that neurons in the rat VPM nucleus receive dense projections from the SI cortex (8, 9) and that these projections are organized in a topographic manner (10). However, the function of these projections has been elusive. In fact, there have been several attempts to unravel the potential physiological contribution of corticofugal projections in a variety of sensory systems. For instance, penicillin-induced epileptic discharge in the cat somatosensory cortex (11) and cortical spreading depression in the rat cortex (12) were found to induce a depression of evoked responses in thalamus. In another series of experiments carried out in both anesthetized and awake preparations, Yuan et al. (13, 14) reported that inactivation of the SI cortex resulted in reduced thalamic responses to electrocutaneous stimulation without any effect on thalamic spontaneous activity, stimulus threshold, response latency, and receptive fields. Other studies, however, have reported a facilitatory influence of the SI cortex on evoked thalamic discharges (15, 16) using cortical spreading depression (17) or electrical stimulation (18). By mapping the effects of stimulation of the SI cortex, Shin and Chapin (19) described a range of thalamic effects, including an overall suppressive influence on thalamic sensory responses, that depended upon the topographic location of the neurons in the ventral posterior nucleus of the thalamus. Recently, Erzenziger et al. (20) reported that chronic manipulations of neuronal activity in the primate primary somatosensory cortex resulted in the expansion of RFs in the VPL nucleus. Elimination of corticofugal projections also led neurons in the spinal subdivision of the trigeminal brainstem complex to increase their responsiveness to whisker stimuli (21). The functions of corticofugal projections have also been investigated in other sensory systems. For instance, in the bat auditory system, focal inactivation of the auditory cortex caused thalamic neurons to shift their tuning curves to different frequencies than the one represented in the inactivated cortical area (22). Finally, Murphy and Sillito (23) reported that cooling of area 17 in cats led neurons located in the lateral geniculate nucleus to lose their well tuned responses to particular lengths of visual stimuli.
Our results indicate that corticothalamic projections not only contribute to the definition of spatiotemporal RFs in the VPM, as hypothesized (24), but also provide a fundamental component that drives the immediate thalamic reorganization observed after a peripheral sensory deafferentation. There are many implications of these results. In terms of the normal functional organization of the somatosensory system, these findings challenge the classical notion that a strict feed-forward labeled-line scheme is used to encode sensory information in this pathway. Rather, our data are more consistent with an alternative model in which cortically originated signals are capable of modulating ascending sensory volleys originating in peripheral receptors (25, 26). This model suggests that the modulation is achieved through integration of activity within intracortical structures that project through descending corticofugal pathways to the thalamus and probably many brainstem nuclei. The present results clearly demonstrate that descending cortical projections influence ascending sensory signals by exerting a dual physiological effect on thalamic neurons. These effects may include a tonic inhibition, generated primarily by cortically driven excitation of GABAergic neurons located in the reticular nucleus of the thalamus and probably in the trigeminal brainstem complex (21, 27). This effect would be consistent with the observation that cortical inactivation triggers unmasking of short-latency responses in the VPM. Moreover, our results indicate that direct corticothalamic glutamatergic projections may account for a significant number of the long-latency excitatory responses of VPM neurons that have been demonstrated to have (in 40% of the VPM neurons) a different RF center (or PW) than the one observed at short latency (6). In this scheme, the spatiotemporal structure of a single VPM neuron RF would result from the temporally asynchronous convergence of parallel ascending trigeminothalamic projections and the dual excitatory and inhibitory effects of descending corticothalamic pathways. Finally, our data provide further support for previous studies that have argued for an important role for thalamic plasticity in cortical reorganization within somatosensory (28) and visual systems (29).
Since the system-wide reorganization reported herein occurs immediately after a peripheral deafferentation, we propose that simple changes in the balance of excitatory and inhibitory influences, produced at each level of the pathway by the dynamic interplay of ascending and descending projections, would explain the changes in neuronal RF and population maps described in this and other studies (3, 4). In fact, the high degree of similarity between cortical and thalamic reorganizations (4) could be explained by the influence of corticofugal projections on neuronal activity within the thalamus or even lower processing stations within the brainstem. In this scenario, changes at the cortical level would be immediately transmitted to large regions of the thalamus and lower brainstem structures. The combination of these descending effects and simultaneous changes in the trigeminothalamic pathways could explain why no clear sequential reorganization can be observed in this system. Instead, both thalamic and cortical reorganization tend to occur essentially simultaneously (within the limits of the temporal resolution of such observations, which is on the order of seconds to minutes) (4). The present results, however, cannot unambiguously determine where within the entire pathway plastic changes might be occurring or the relative contribution of excitatory or inhibitory inputs. Further work will be required to resolve the details of these processes.
Overall, our results imply that both the normal encoding of tactile information and the process of injury-dependent plastic reorganization depend on system-wide interactions that involve many brain structures and can only be fully reconstructed (or decoded) by examining the simultaneous dynamic interactions between populations of cortical and subcortical neurons.
Acknowledgments
We thank Drs. A. J. Brisben and C. G. Logan for critical comments and suggestions during preparation of this manuscript.
ABBREVIATIONS
- RF
receptive field
- SI
primary somatosensory
- VPM
ventroposterior medial thalamus
- PW
principal whisker
Footnotes
A Commentary on this article begins on page 7622.
References
- 1.Merzenich M M, Kaas J H, Wall J T, Sur M, Nelson R J, Felleman D J. Neuroscience. 1983;10:639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- 2.Calford M B, Tweedale R. Nature (London) 1988;332:446–448. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- 3.Nicolelis M A, Lin R C, Woodward D J, Chapin J K. Nature (London) 1993;361:533–536. doi: 10.1038/361533a0. [DOI] [PubMed] [Google Scholar]
- 4.Faggin B M, Nguyen K T, Nicolelis M A. Proc Natl Acad Sci USA. 1997;94:9428–9433. doi: 10.1073/pnas.94.17.9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolelis M A. Semin Neurosci. 1997;9:24–33. [Google Scholar]
- 6.Nicolelis M A, Chapin J K. J Neurosci. 1994;14:3511–3532. doi: 10.1523/JNEUROSCI.14-06-03511.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolelis M A, Ghazanfar A A, Faggin B M, Votaw S, Oliveira L M. Neuron. 1997;18:529–537. doi: 10.1016/s0896-6273(00)80295-0. [DOI] [PubMed] [Google Scholar]
- 8.Bourassa J, Pinault D, Deschenes M. Eur J Neurosci. 1995;7:19–30. doi: 10.1111/j.1460-9568.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 9.Chmielowska J, Carvell G E, Simons D J. J Comp Neurol. 1989;285:325–338. doi: 10.1002/cne.902850304. [DOI] [PubMed] [Google Scholar]
- 10.Hoogland P V, Welker E, Van der Loos H. Exp Brain Res. 1987;68:73–87. doi: 10.1007/BF00255235. [DOI] [PubMed] [Google Scholar]
- 11.Ogden T E. Electroenceph Clin Neurophysiol. 1960;12:621–634. doi: 10.1016/0013-4694(60)90106-1. [DOI] [PubMed] [Google Scholar]
- 12.Albe-Fessard D, Condes-Lara M, Kesar S, Sanderson P. In: Somatosensory Integration in the Thalamus. Macchi G, Rustioni A, Speafico R, editors. Amsterdam: Elsevier; 1983. pp. 273–285. [Google Scholar]
- 13.Yuan B, Morrow T J, Casey K L. J Neurosci. 1986;6:3611–3617. doi: 10.1523/JNEUROSCI.06-12-03611.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan B, Morrow T J, Casey K L. J Neurosci. 1985;5:2971–2978. doi: 10.1523/JNEUROSCI.05-11-02971.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen P, Junge K, Sveen O. Nature (London) 1967;214:1011–1012. doi: 10.1038/2141011a0. [DOI] [PubMed] [Google Scholar]
- 16.Andersen P, Junge K, Sveen O. Brain Behav Evol. 1972;6:170–184. doi: 10.1159/000123705. [DOI] [PubMed] [Google Scholar]
- 17.Waller H J, Feldman S M. Science. 1967;157:1074–1077. doi: 10.1126/science.157.3792.1074. [DOI] [PubMed] [Google Scholar]
- 18.Anderson P, Eccles J C, Sears T A. J Neurophys. 1964;27:63–77. doi: 10.1152/jn.1964.27.1.63. [DOI] [PubMed] [Google Scholar]
- 19.Shin H C, Chapin J K. Somatosens Mot Res. 1990;7:421–434. doi: 10.3109/08990229009144717. [DOI] [PubMed] [Google Scholar]
- 20.Ergenzinger E R, Glasier M M, Hahm J O, Pons T P. Nature Neurosci. 1998;1:226–229. doi: 10.1038/673. [DOI] [PubMed] [Google Scholar]
- 21.Jacquin M F, Wiegand M R, Renehan W E. J Neurophysiol. 1990;64:3–27. doi: 10.1152/jn.1990.64.1.3. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Suga N, Yan J. Nature (London) 1997;387:900–903. doi: 10.1038/43180. [DOI] [PubMed] [Google Scholar]
- 23.Murphy P C, Sillito A M. Nature (London) 1987;329:727–729. doi: 10.1038/329727a0. [DOI] [PubMed] [Google Scholar]
- 24.Ghazanfar A A, Krupa D J, Nicolelis M A L. Soc Neurosci Abstracts. 1997;23:1797. (abstract). [Google Scholar]
- 25.Chapin J K, Woodward D J. Exp Neurol. 1981;72:164–178. doi: 10.1016/0014-4886(81)90135-7. [DOI] [PubMed] [Google Scholar]
- 26.Chapin J K, Woodward D J. Exp Neurol. 1982;78:670–684. doi: 10.1016/0014-4886(82)90083-8. [DOI] [PubMed] [Google Scholar]
- 27.Lee S M, Friedberg M H, Ebner F F. J Neurophysiol. 1994;71:1702–1715. doi: 10.1152/jn.1994.71.5.1702. [DOI] [PubMed] [Google Scholar]
- 28.Garraghty P E, Kaas J H. Neuroreport. 1991;2:747–750. doi: 10.1097/00001756-199112000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Eysel U T. Nature (London) 1982;299:442–444. doi: 10.1038/299442a0. [DOI] [PubMed] [Google Scholar]