Abstract
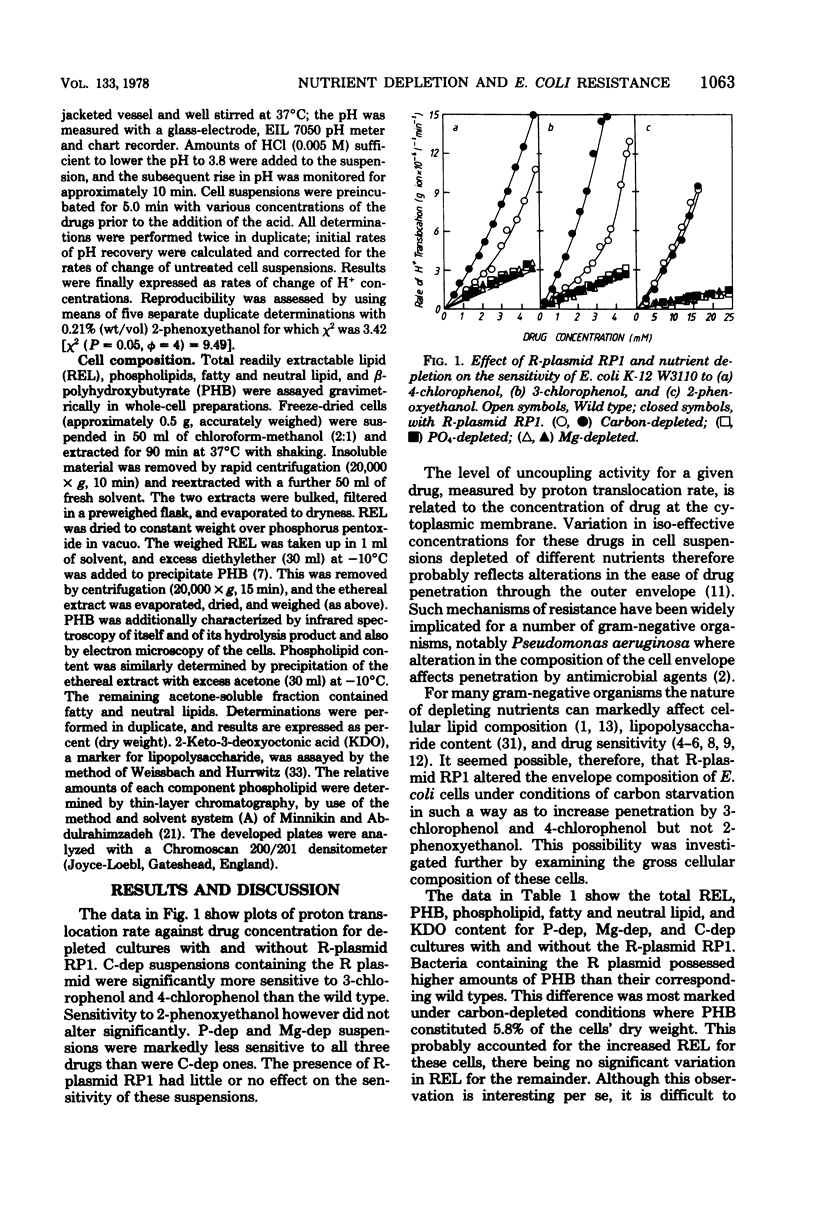
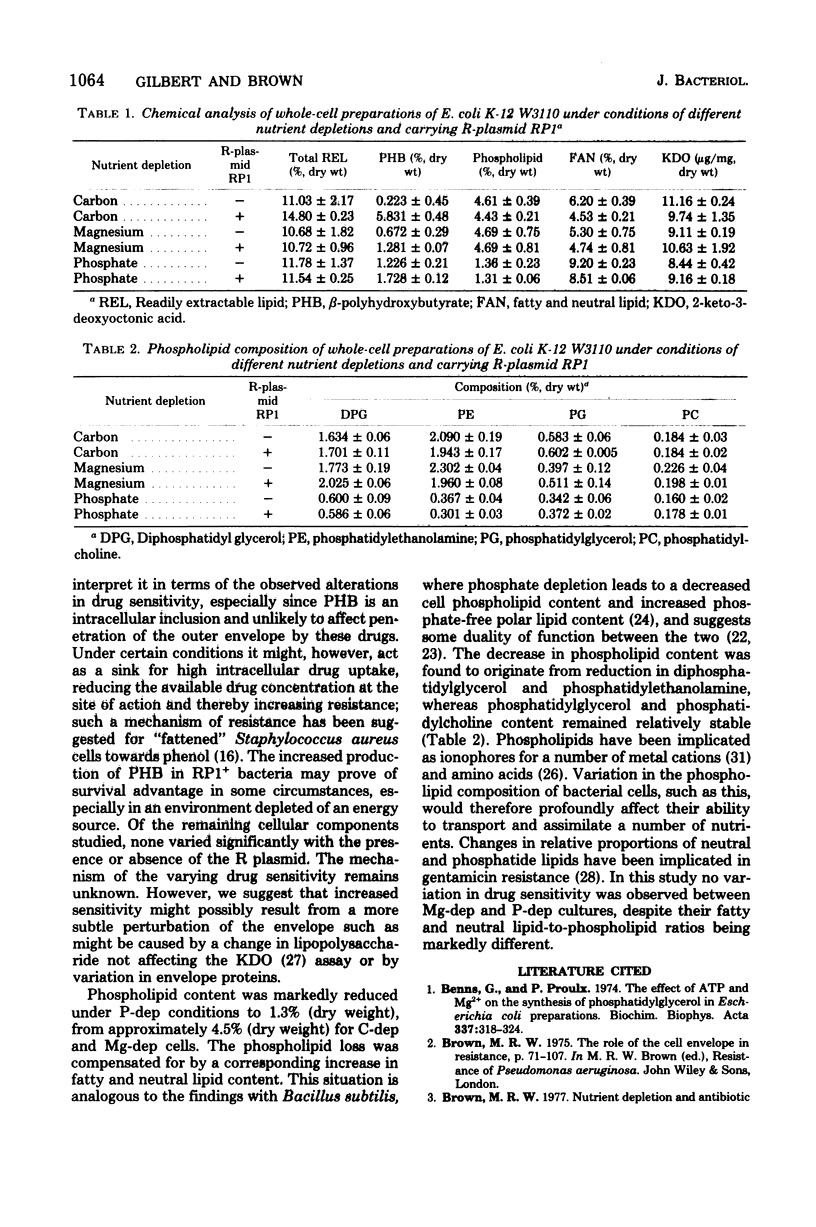
The resistance of Escherichia coli batch cultures depleted of carbon (C-dep), magnesium (Mg-dep), or phosphate (P-dep) against low concentrations of 3-chlorophenol, 4-chlorophenol, or 2-phenoxyethanol varied. C-dep cultures were always significantly more sensitive than Mg-dep or P-dep cultures. The presence of R-plasmid RP1 increased the sensitivity of C-dep cultures to 3- and 4-chlorophenol, yet had little effect on those cultured depleted in magnesium or phosphate ions. Cultures with R-plasmid RP1 had increased levels of beta-polyhydroxybutyrate irrespective of the nature of the depleting nutrient. P-dep bacteria had less than one-third of the phospholipid of other cell types, this deficiency being compensated for by increases in fatty acid and neutral lipid content. The reduction in phospholipid content of P-dep cultures was entirely accounted for by decreased diphosphatidylglycerol and phosphatidylethanolamine levels in these cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benns G., Proulx P. The effect of ATP and Mg2+ on the synthesis of phosphatidylglycerol in Escherichia coli preparations. Biochim Biophys Acta. 1974 Mar 28;337(3):318–324. doi: 10.1016/0005-2760(74)90106-4. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Anderson R. A. The bactericidal effect of silver ions on Pseudomonas aeruginosa. J Pharm Pharmacol. 1968 Dec;20(Suppl):1S+–1S+. doi: 10.1111/j.2042-7158.1968.tb09850.x. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Melling J. Loss of sensitivity to EDTA by Pseudomonas aeruginosa grown under conditions of Mg-limitation. J Gen Microbiol. 1968 Dec;54(3):439–444. doi: 10.1099/00221287-54-3-439. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Melling J. Role of divalent cations in the action of polymyxin B and EDTA on Pseudomonas aeruginosa. J Gen Microbiol. 1969 Dec;59(2):263–274. doi: 10.1099/00221287-59-2-263. [DOI] [PubMed] [Google Scholar]
- Brown M. R. Nutrient depletion and antibiotic susceptibility. J Antimicrob Chemother. 1977 May;3(3):198–201. doi: 10.1093/jac/3.3.198. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Watkins W. M. Low magnesium and phospholipid content of cell wals of Pseudomonas aeruginosa resistant to polymyxin. Nature. 1970 Sep 26;227(5265):1360–1361. doi: 10.1038/2271360a0. [DOI] [PubMed] [Google Scholar]
- Finch J. E., Brown M. R. The influence of nutrient limitation in a chemostat on the sensitivity of Pseudomonas aeruginosa to polymyxin and to EDTA. J Antimicrob Chemother. 1975 Dec;1(4):379–386. doi: 10.1093/jac/1.4.379. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Eagon R. G. Ultrastructural and chemical alteration of the cell envelope of Pseudomonas aeruginosa, associated with resistance to ethylenediaminetetraacetate resulting from growth in a Mg2+-deficient medium. J Bacteriol. 1974 Jan;117(1):302–311. doi: 10.1128/jb.117.1.302-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther T., Richter L., Schmalbeck J. Phospholipids of Escherichia coli in magnesium deficiency. J Gen Microbiol. 1975 Jan;86(1):191–193. doi: 10.1099/00221287-86-1-191. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M., O'Hara K. Mechanism of chloramphenicol-resistance mediated by kR102 factor in Pseudomonas aeruginosa. J Antibiot (Tokyo) 1976 Feb;29(2):176–180. doi: 10.7164/antibiotics.29.176. [DOI] [PubMed] [Google Scholar]
- Lowbury E. J., Lilly H. A., Kidson A., Ayliffe G. A., Jones R. J. Sensitivity of Pseudomonas aeruginosa to antibiotics: emergence of strains highly resistant to carbenicillin. Lancet. 1969 Aug 30;2(7618):448–452. doi: 10.1016/s0140-6736(69)90163-9. [DOI] [PubMed] [Google Scholar]
- Meers J. L., Tempest D. W. The influence of growth-limiting substrate and medium NaCl concentration on the synthesis of magnesium-binding sites in the walls of Bacillus subtilis var. niger. J Gen Microbiol. 1970 Nov;63(3):325–331. doi: 10.1099/00221287-63-3-325. [DOI] [PubMed] [Google Scholar]
- Minnikin D. E., Abdolrahimzadeh H., Baddiley J. The interrelation of phosphatidylethanolamine and glycosyl diglycerides in bacterial membranes. Biochem J. 1971 Sep;124(2):447–448. doi: 10.1042/bj1240447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnikin D. E., Abdolrahimzadeh H., Baddiley J. The interrelation of polar lipids in bacterial membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):651–655. doi: 10.1016/0005-2736(71)90148-9. [DOI] [PubMed] [Google Scholar]
- Minnikin D. E., Abdolrahimzadeh H., Baddiley J. Variation of polar lipid composition of Bacillus subtilis (Marburg) with different growth conditions. FEBS Lett. 1972 Oct 15;27(1):16–18. doi: 10.1016/0014-5793(72)80398-3. [DOI] [PubMed] [Google Scholar]
- Minnikin D. E., Abdolrahimzadeh H. Thin-layer chromatography of bacterial lipids on sodium acetate-impregnated silica gel. J Chromatogr. 1971 Dec 23;63(2):452–454. doi: 10.1016/s0021-9673(01)85672-7. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T., Okuda S., Takahashi H. Relationship between phospholipid compositions and transport activities of amino acids in Escherichia coli membrane vesicles. Biochim Biophys Acta. 1977 Apr 1;466(1):44–56. doi: 10.1016/0005-2736(77)90207-3. [DOI] [PubMed] [Google Scholar]
- Pazoles C. J., Kulpa C. F., Jr Biosynthesis and structure of lipopolysaccharide in an outer membrane-defective mutant of Escherichia coli J5. Biochim Biophys Acta. 1977 Apr 1;466(1):160–175. doi: 10.1016/0005-2736(77)90216-4. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Ellwood D. C. The influence of growth conditions on the composition of some cell wall components of Aerobacter aerogenes. Biotechnol Bioeng. 1969 Sep;11(5):775–783. doi: 10.1002/bit.260110507. [DOI] [PubMed] [Google Scholar]
- Tyson C. A., Vande Zande H., Green D. E. Phospholipids as ionophores. J Biol Chem. 1976 Mar 10;251(5):1326–1332. [PubMed] [Google Scholar]
- WEINBACH E. C., GARBUS J. THE INTERACTION OF UNCOUPLING PHENOLS WITH MITOCHONDRIA AND WITH MITOCHONDRIAL PROTEIN. J Biol Chem. 1965 Apr;240:1811–1819. [PubMed] [Google Scholar]


