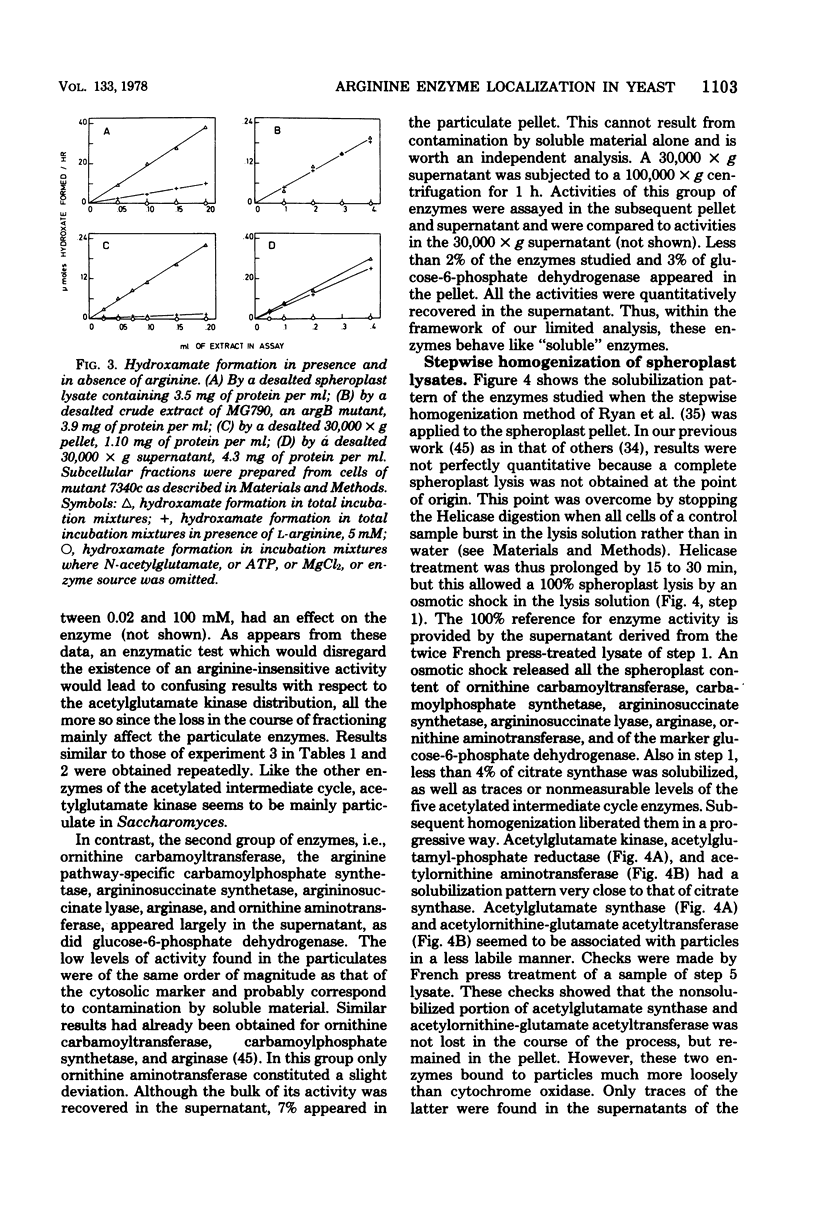
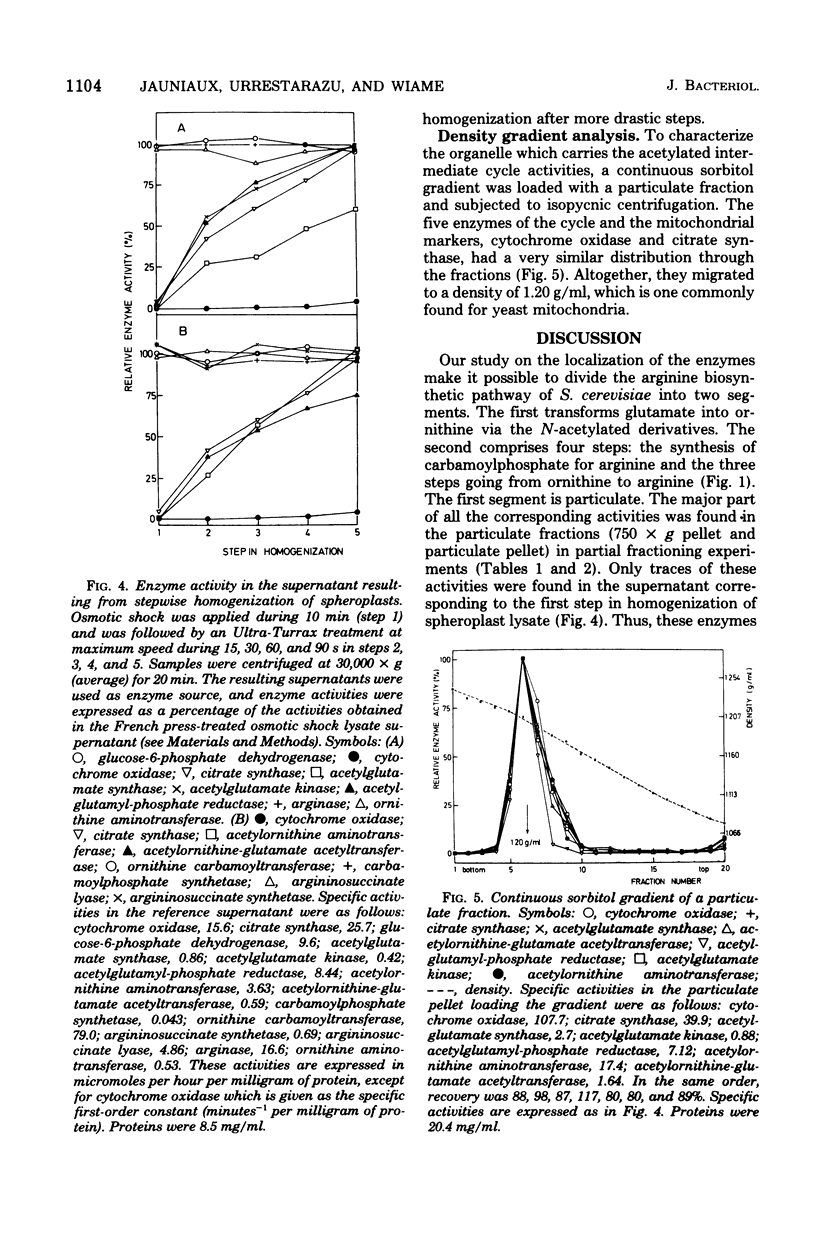
Abstract
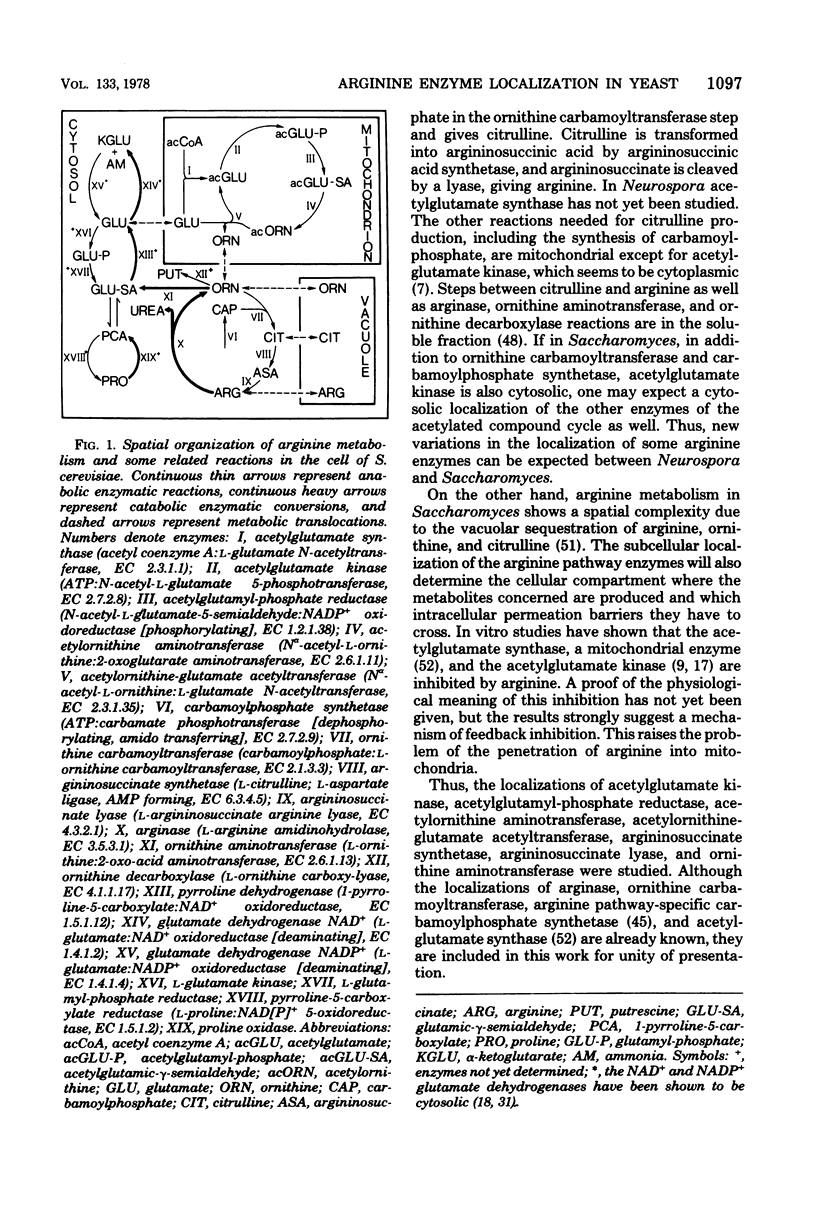
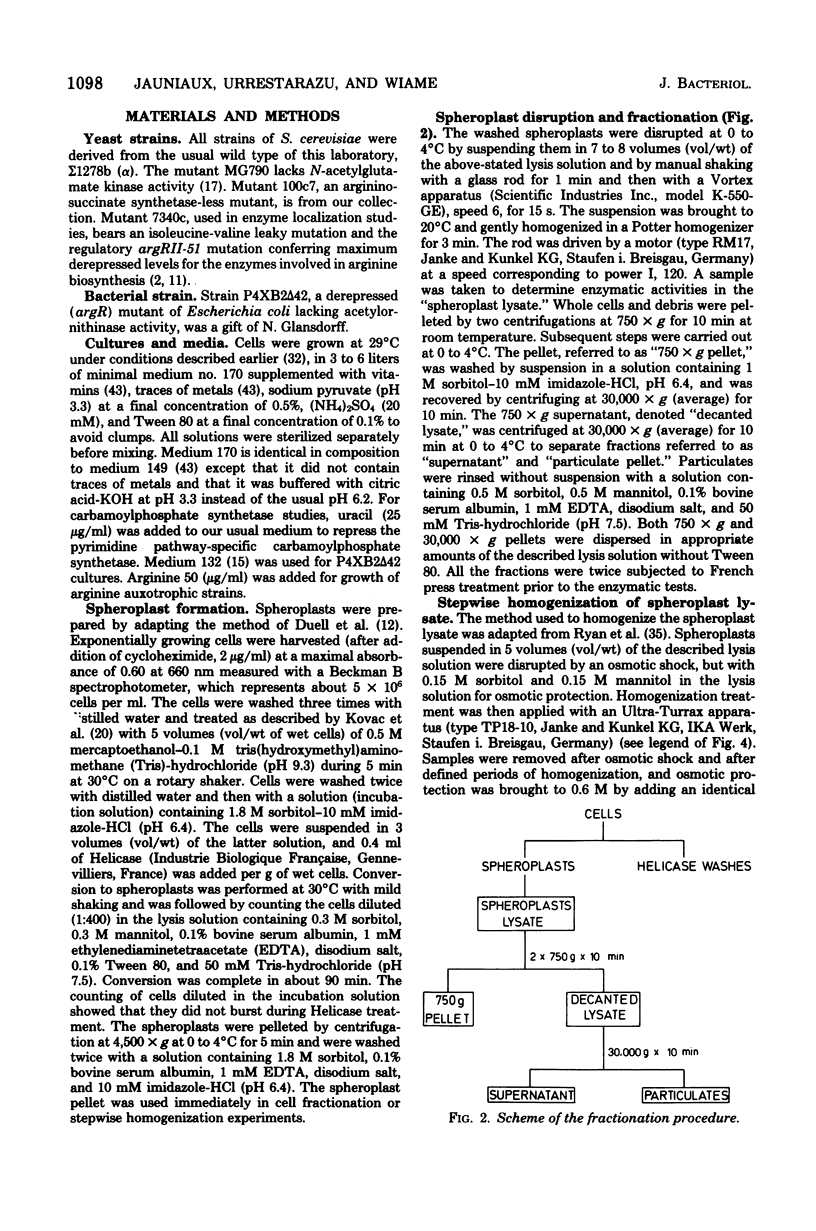
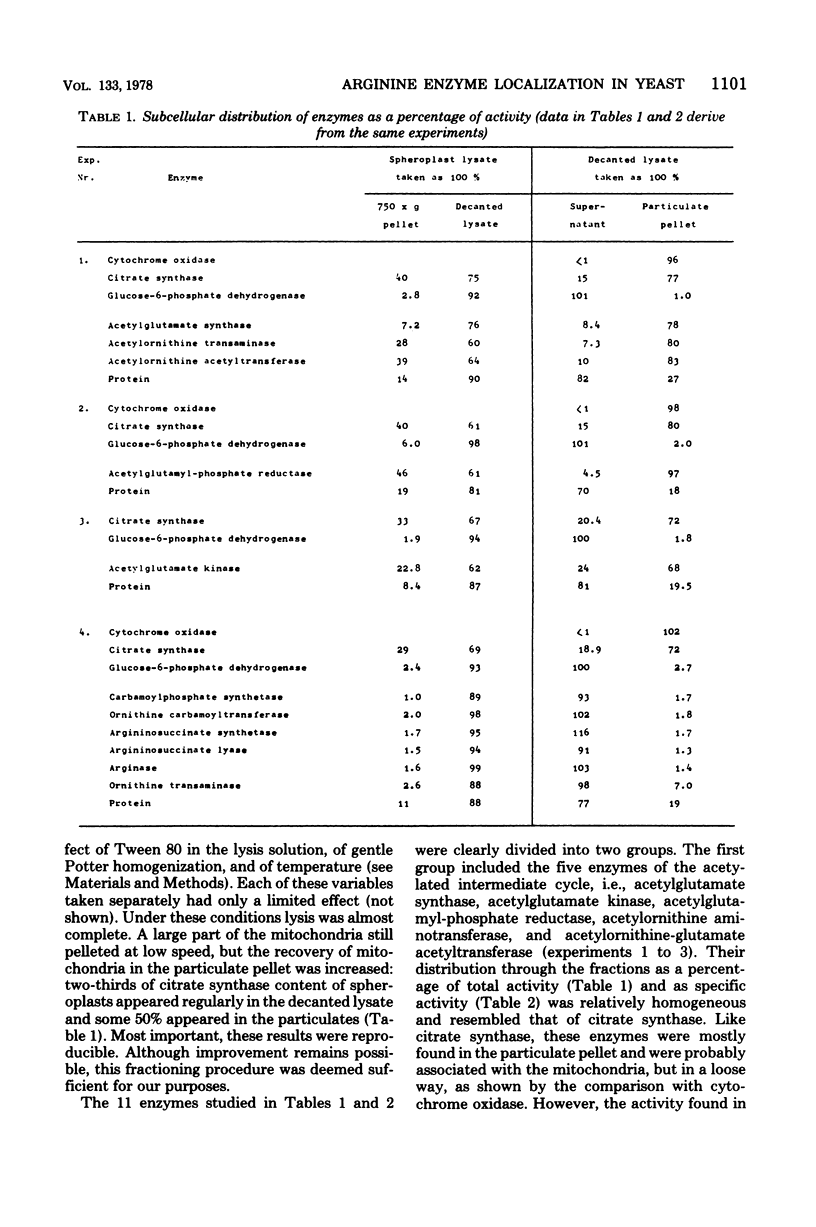
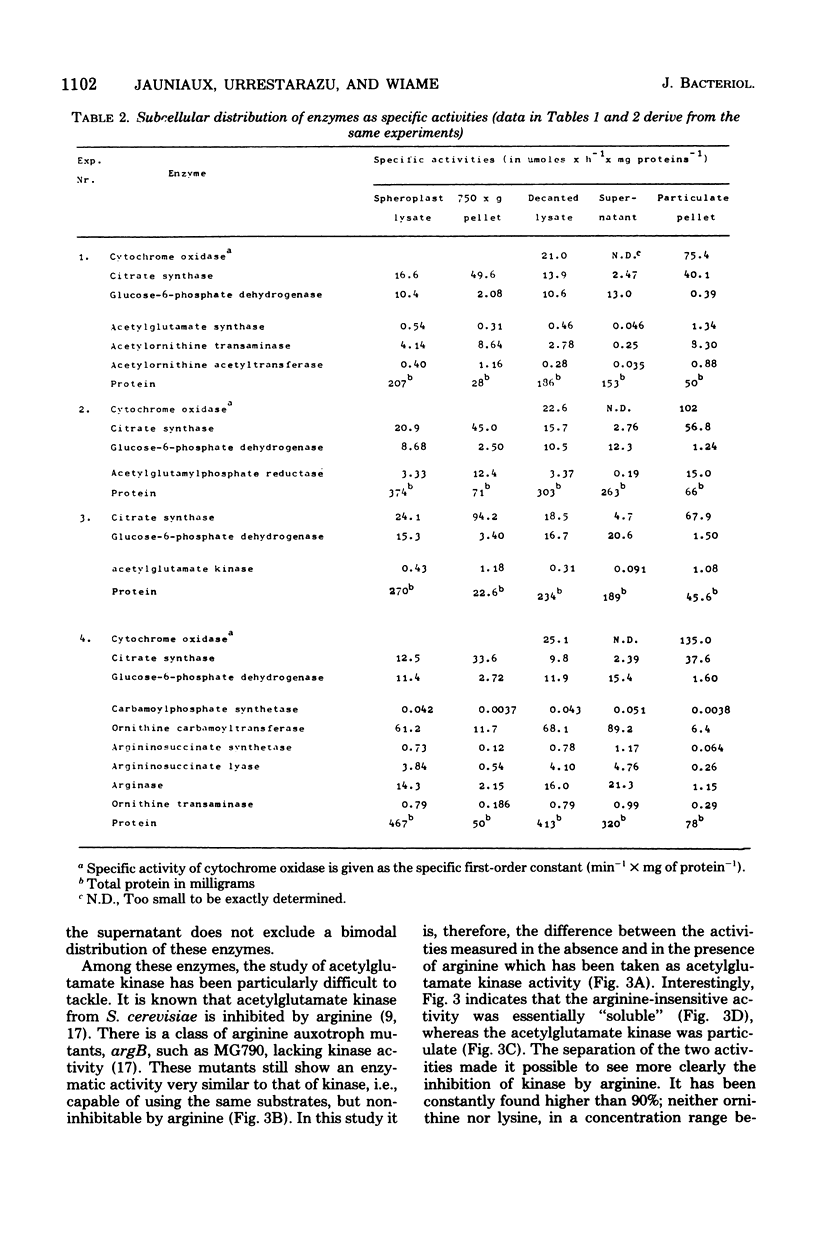
Subcellular localization of enzymes of arginine metabolism in Saccharomyces cerevisiae was studied by partial fractionation and stepwise homogenization of spheroplast lysates. These enzymes could clearly be divided into two groups. The first group comprised the five enzymes of the acetylated compound cycle, i.e., acetylglutamate synthase, acetylglutamate kinase, acetylglutamyl-phosphate reductase, acetylornithine aminotransferase, and acetylornithine-glutamate acetyltransferase. These enzymes were exclusively particulate. Comparison with citrate synthase and cytochrome oxidase, and results from isopycnic gradient analysis, suggested that these enzymes were associated with the mitochondria. By contrast, enzymatic activities going from ornithine to arginine, i.e., arginine pathway-specific carbamoylphosphate synthetase, ornithine carbamoyltransferase, argininosuccinate synthetase, and argininosuccinate lyase, and the two first catabolic enzymes, arginase and ornithine aminotransferase, were in the "soluble" fraction of the cell.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bechet J., Greenson M., Wiame J. M. Mutations affecting the repressibility of arginine biosynthetic enzymes in Saccharomyces cerevisiae. Eur J Biochem. 1970 Jan;12(1):31–39. doi: 10.1111/j.1432-1033.1970.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Boller T., Dürr M., Wiemken A. Characterization of a specific transport system for arginine in isolated yeast vacuoles. Eur J Biochem. 1975 May;54(1):81–91. doi: 10.1111/j.1432-1033.1975.tb04116.x. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Davis R. H. Arginine catabolism in Neurospora: cycling of ornithine. J Bacteriol. 1977 Apr;130(1):285–291. doi: 10.1128/jb.130.1.285-291.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Davis R. H. Cellular distribution of ornithine in Neurospora: anabolic and catabolic steady states. J Bacteriol. 1977 Apr;130(1):274–284. doi: 10.1128/jb.130.1.274-284.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybis J., Davis R. H. Organization and control in the arginine biosynthetic pathway of Neurospora. J Bacteriol. 1975 Jul;123(1):196–202. doi: 10.1128/jb.123.1.196-202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DEKEN R. H. Pathway of arginine biosynthesis in yeast. Biochem Biophys Res Commun. 1962 Aug 31;8:462–466. doi: 10.1016/0006-291x(62)90297-8. [DOI] [PubMed] [Google Scholar]
- DUELL E. A., INOUE S., UTTER M. F. ISOLATION AND PROPERTIES OF INTACT MITOCHONDRIA FROM SPHEROPLASTS OF YEAST. J Bacteriol. 1964 Dec;88:1762–1773. doi: 10.1128/jb.88.6.1762-1773.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H. Metabolite distribution in cells. Science. 1972 Nov 24;178(4063):835–840. doi: 10.1126/science.178.4063.835. [DOI] [PubMed] [Google Scholar]
- Delforge J., Messenguy F., Wiame J. M. The regulation of arginine biosynthesis in Saccharomyces cerevisiae. The specificity of argR- mutations and the general control of amino-acid biosynthesis. Eur J Biochem. 1975 Sep 1;57(1):231–239. doi: 10.1111/j.1432-1033.1975.tb02295.x. [DOI] [PubMed] [Google Scholar]
- Fukui S., Kawamoto S., Yasuhara S., Tanaka A., Osumi M. Microbody of methanol-grown yeasts. Localization of catalase and flavin-dependent alcohol oxidase in the isolated microbody. Eur J Biochem. 1975 Nov 15;59(2):561–566. doi: 10.1111/j.1432-1033.1975.tb02482.x. [DOI] [PubMed] [Google Scholar]
- GLANSDORFF N. TOPOGRAPHY OF COTRANSDUCIBLE ARGININE MUTATIONS IN ESCHERICHIA COLI K-12. Genetics. 1965 Feb;51:167–179. doi: 10.1093/genetics/51.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J. G., Lehninger A. L. Transport of ornithine and citrulline across the mitochondrial membrane. J Biol Chem. 1973 Jan 25;248(2):610–618. [PubMed] [Google Scholar]
- Haas D., Kurer V., Leisinger T. N-acetylglutamate synthetase of Pseudomonas aeruginosa. An assay in vitro and feedback inhibition by arginine. Eur J Biochem. 1972 Dec 4;31(2):290–295. doi: 10.1111/j.1432-1033.1972.tb02531.x. [DOI] [PubMed] [Google Scholar]
- Hilger F., Culot M., Minet M., Pierard A., Grenson M., Wiame J. M. Studies on the kinetics of the enzyme sequence mediating arginine synthesis in Saccharomyces cerevisiae. J Gen Microbiol. 1973 Mar;75(1):33–41. doi: 10.1099/00221287-75-1-33. [DOI] [PubMed] [Google Scholar]
- Hollenberg C. P., Riks W. F., Borst P. The glutamate dehydrogenases of yeast: extra-mitochondrial enzymes. Biochim Biophys Acta. 1970 Jan 27;201(1):13–19. doi: 10.1016/0304-4165(70)90004-8. [DOI] [PubMed] [Google Scholar]
- Kohlhaw G. B., Tan-Wilson A. Carnitine acetyltransferase: candidate for the transfer of acetyl groups through the mitochondrial membrane of yeast. J Bacteriol. 1977 Feb;129(2):1159–1161. doi: 10.1128/jb.129.2.1159-1161.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacroute F., Piérard A., Grenson M., Wiame J. M. The biosynthesis of carbamoyl phosphate in Saccharomyces cerevisiae. J Gen Microbiol. 1965 Jul;40(1):127–142. doi: 10.1099/00221287-40-1-127. [DOI] [PubMed] [Google Scholar]
- Messenguy F., Penninckx M., Wiame J. M. Interaction between arginase and ornithine carbamoyltransferase in Saccharomyces cerevisiae. The regulatory site for ornithine. Eur J Biochem. 1971 Sep 24;22(2):277–286. doi: 10.1111/j.1432-1033.1971.tb01542.x. [DOI] [PubMed] [Google Scholar]
- Messenguy F., Wiame J. -M. The control of ornithinetranscarbamylase activity by arginase in Saccharomyces cerevisiae. FEBS Lett. 1969 Apr;3(1):47–49. doi: 10.1016/0014-5793(69)80093-1. [DOI] [PubMed] [Google Scholar]
- Parish R. W. The isolation and characterization of peroxisomes (microbodies) from baker's yeast, Saccharomyces cerevisiae. Arch Microbiol. 1975 Nov 7;105(3):187–192. doi: 10.1007/BF00447136. [DOI] [PubMed] [Google Scholar]
- Penninckx M., Simon J. P., Wiame J. M. Interaction between arginase and L-ornithine carbamoyltransferase in Saccharomyces cerevisiae. Purification of S. cerevisiae enzymes and evidence that these enzymes as well as rat-liver arginase are trimers. Eur J Biochem. 1974 Nov 15;49(2):429–442. doi: 10.1111/j.1432-1033.1974.tb03848.x. [DOI] [PubMed] [Google Scholar]
- Penninckx M., Wiame J. M. Affinity of arginase for ornithine carbamoyltransferase in Saccharomyces cerevisiae. J Mol Biol. 1976 Jul 15;104(4):819–831. doi: 10.1016/0022-2836(76)90184-4. [DOI] [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Intracellular localization of enzymes in yeast. Arch Biochem Biophys. 1970 Jan;136(1):245–259. doi: 10.1016/0003-9861(70)90348-6. [DOI] [PubMed] [Google Scholar]
- Ramos F., Thuriaux P., Wiame J. M., Bechet J. The participation of ornithine and citrulline in the regulation of arginine metabolism in Saccharomyces cerevisiae. Eur J Biochem. 1970 Jan;12(1):40–47. doi: 10.1111/j.1432-1033.1970.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Ryan E. D., Kohlhaw G. B. Subcellular localization of isoleucine-valine biosynthetic enzymes in yeast. J Bacteriol. 1974 Nov;120(2):631–637. doi: 10.1128/jb.120.2.631-637.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan E. D., Tracy J. W., Kohlhaw G. B. Subcellular localization of the leucine biosynthetic enzymes in yeast. J Bacteriol. 1973 Oct;116(1):222–225. doi: 10.1128/jb.116.1.222-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELS A. A. [Determination of hemoproteins in yeast. 4. Cytochrome oxidase]. Arch Int Physiol Biochim. 1962 Dec;70:760–762. [PubMed] [Google Scholar]
- SMITH L. A study of some oxidative enzymes of baker's yeast. Arch Biochem Biophys. 1954 Jun;50(2):285–298. doi: 10.1016/0003-9861(54)90044-2. [DOI] [PubMed] [Google Scholar]
- Satyanarayana T., Klein H. P. Studies on acetyl-coenzyme A synthetase of yeast: inhibition by long-chain acyl-coenzyme A esters. J Bacteriol. 1973 Aug;115(2):600–606. doi: 10.1128/jb.115.2.600-606.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana T., Klein H. P. Studies on the "aerobic" acetyl-coenzyme A synthetase of Saccharomyces cerevisiae: purification, crystallization, and physical properties of the enzyme. Arch Biochem Biophys. 1976 Jun;174(2):480–490. doi: 10.1016/0003-9861(76)90376-3. [DOI] [PubMed] [Google Scholar]
- Shigesada K., Tatibana M. Enzymatic synthesis of acetylglutamate by mammalian liver preparations and its stimulation by arginine. Biochem Biophys Res Commun. 1971 Sep;44(5):1117–1124. doi: 10.1016/s0006-291x(71)80201-2. [DOI] [PubMed] [Google Scholar]
- Shigesada K., Tatibana M. Role of acetylglutamate in ureotelism. I. Occurrence and biosynthesis of acetylglutamate in mouse and rat tissues. J Biol Chem. 1971 Sep 25;246(18):5588–5595. [PubMed] [Google Scholar]
- Thuriaux P., Ramos F., Piérard A., Grenson M., Wiame J. M. Regulation of the carbamoylphosphate synthetase belonging to the arginine biosynthetic pathway of Saccharomyces cerevisiae. J Mol Biol. 1972 Jun 20;67(2):277–287. doi: 10.1016/0022-2836(72)90241-0. [DOI] [PubMed] [Google Scholar]
- Tracy J. W., Kohlhaw G. B. Reversible, coenzyme-A-mediated inactivation of biosynthetic condensing enzymes in yeast: a possible regulatory mechanism. Proc Natl Acad Sci U S A. 1975 May;72(5):1802–1806. doi: 10.1073/pnas.72.5.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrestarazu L. A., Vissers S., Wiame J. M. Change in location of ornithine carbamoyltransferase and carbamoylphosphate synthetase among yeasts in relation to the arginase/ornithine carbamoyltransferase regulatory complex and the energy status of the cells. Eur J Biochem. 1977 Oct 3;79(2):473–481. doi: 10.1111/j.1432-1033.1977.tb11830.x. [DOI] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]
- Wiemken A., Dürr M. Characterization of amino acid pools in the vacuolar compartment of Saccharomyces cerevisiae. Arch Microbiol. 1974;101(1):45–57. doi: 10.1007/BF00455924. [DOI] [PubMed] [Google Scholar]


