Abstract
HIV-1 glycoprotein gp120 induces injury and apoptosis in rodent and human neurons in vitro and in vivo and is therefore thought to contribute to HIV-associated dementia. In addition to CD4, different gp120 isolates bind to the α- or β-chemokine receptors CXCR4 and CCR5, respectively. These and other chemokine receptors are on brain macrophages/microglia, astrocytes, and neurons. Thus, apoptosis could occur via direct interaction of gp120 with neurons, indirectly via stimulation of glia to release neurotoxic factors, or via both pathways. Here we show in rat cerebrocortical cultures that recapitulate the type and proportion of cells normally found in brain, i.e., neurons, astrocytes, and macrophages/microglia, that the β-chemokines RANTES (regulated on activation, normal T cell expressed and secreted) and macrophage inflammatory protein (MIP-1β) protect neurons from gp120SF2-induced apoptosis. The gp120SF2 isolate prefers binding to CXCR4 receptors, similar to the physiological α-chemokine ligands, stromal cell-derived factor (SDF)-1α/β. SDF-1α/β failed to prevent gp120SF2 neurotoxicity, and in fact also induced neuronal apoptosis. We could completely abrogate gp120SF2-induced neuronal apoptosis with the tripeptide TKP, which inhibits activation of macrophages/microglia. In contrast, TKP or depletion of macrophages/microglia did not prevent SDF-1 neurotoxicity. Inhibition of p38 mitogen-activated protein kinase ameliorated both gp120SF2- and SDF-1-induced neuronal apoptosis. Taken together, these results suggest that gp120SF2 and SDF-1 differ in the cell type on which they stimulate CXCR4 to induce neuronal apoptosis, but both ligands use the p38 mitogen-activated protein kinase pathway for death signaling. Moreover, gp120SF2-induced neuronal apoptosis depends predominantly on an indirect pathway via activation of chemokine receptors on macrophages/microglia, whereas SDF-1 may act directly on neurons or astrocytes.
About half of children and a quarter of adults infected with HIV-1 eventually develop dementia (1). Transgenic mice expressing the HIV-1 envelope glycoprotein gp120 manifest neuropathological features that resemble in many ways the findings in brains of AIDS patients (2). In vitro and in vivo, gp120 produces injury and apoptosis in both primary rodent and human neurons (3–9). Recent evidence has shown that gp120 binds, respectively, to macrophages and T cells via the chemokine receptors CCR5 and CXCR4, which, in addition to CD4, function as coreceptors for HIV-1 (10–13). Nonetheless, CCR5 and CXCR4, as well as other chemokine receptors, are also present on neurons and astrocytes (12, 14–16). Thus, a major question addressed in the present study is whether gp120-induced neuronal injury occurs as a consequence of direct interaction with neurons via chemokine receptors and their cognate G protein-signaling systems (13, 17) or indirectly via the release of macrophage toxic factors, as previously suggested from in vitro experiments with gp120-conditioned medium after macrophage depletion (18–22). Finally, both direct and indirect pathways in conjunction could contribute to neuronal death, in a manner similar to that recently shown for the CXCR4-mediated killing of CD8+ T cells (23). Although some gp120 variants can signal via chemokine receptors on neuronal cell lines and on isolated rodent neurons (13, 17), the importance of cell–cell interactions in the brain mandates that disease pathogenesis in vitro be approached in a culture system that recapitulates the type and proportion of cells normally found in brain, i.e., neurons, astrocytes, and macrophages/microglia.
Here we show in such a “mixed” culture system (24) that the β-chemokines RANTES (regulated on activation, normal T cell expressed and secreted) or MIP-1β can protect rat cerebrocortical neurons from gp120-induced apoptosis, whereas the α- chemokines SDF-1α and β not only fail to prevent gp120 neurotoxicity but induce neuronal apoptosis themselves. The tuftsin-derived tripeptide TKP (Thr-Lys-Pro), which inhibits macrophage/microglial activation (25–28), completely abrogates gp120-induced neuronal apoptosis. In contrast, TKP or depletion of monocytoid cells from the culture does not prevent the neurotoxicity of SDF-1, indicating that it is independent of macrophages/microglia. However, inhibition of the p38 mitogen-activated protein kinase (MAPK) signaling pathway ameliorates both gp120- and SDF-1-induced neuronal damage. Thus, gp120SF2 and SDF-1 stimulate CXCR4 receptors on different cell types; yet in both cases, p38 MAPK is in the signaling pathway to neuronal apoptosis. Additionally, our results suggest that gp120SF2-induced neuronal apoptosis is mediated indirectly via chemokine receptors on macrophages/microglia, whereas the α-chemokines SDF-1α and β appear to exert their action directly on neurons or astrocytes.
MATERIALS AND METHODS
Peptides and Recombinant Proteins.
The tripeptide TKP (Thr-Lys-Pro; tuftsin fragment 1–3) was obtained from Sigma. Recombinant human MIP-1β, SDF-1α, SDF-1β, and recombinant rat RANTES were purchased from R&D Systems and Endogen (Cambridge, MA), respectively. HIV-1 envelope glycoprotein gp120 from the strain SF2 was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (29). Additional gp120s from HIV-1 strains IIIB and RF2 were obtained from Genentech and the National Cancer Institute, respectively, and in previous experiments were found to produce neurotoxicity similar to gp120SF2 (4, 18, 21, 30–33). Tumor necrosis factor α, IFN-γ, and IL-1β were from Genzyme, GIBCO/BRL, and Endogen (Cambridge, MA), respectively.
Preparation and Treatment of Rat Cerebrocortical Cultures.
Cerebrocortical cultures were prepared from embryos of Sprague–Dawley rats at day 15–17 of gestation, as described (34, 35). Cultures were used for experiments after 17–24 days in culture. These cultures contain neurons, astrocytes, and macrophages/microglia, as determined with specific immunolabeling. Before some experiments, macrophages/microglia or neurons were depleted from the cultures by exposure to 7.5 mM l-leucine methyl ester or 2 mM N-methyl-d-aspartate (NMDA), respectively (18, 34). Absence of macrophages/microglia or neurons in these cultures was confirmed immunocytochemically, by using antibodies to ED-1 and microtubule-associated protein-2 (MAP-2), respectively. In some experiments, the Griess reaction was used to measure nitrite levels in the culture medium as an index of NO release (36).
Incubation of Cells with TKP, Chemokines, and p38 MAPK Inhibitor.
Cultures were transferred into Earle’s balanced salt solution and incubated for 24 hr with gp120, chemokines, TKP, p38 MAPK inhibitor SB203580 (Calbiochem), or combinations thereof. Chemokines or TKP were applied for 5 min and SB203580 for 15 min before gp120 exposure.
Assessment of Neuronal Apoptosis.
Apoptosis in these cultures was assessed by using multiple methods with concordant results as detailed (24). We routinely used a combination of staining of permeabilized cells with propidium iodide to determine apoptotic morphology and a neuron-specific antibody to identify cell type. In brief, cells were fixed for 5 min with ice-cold acetone at −20°C and, after three washes in PBS, for 4 min with 2% (wt/vol) paraformaldehyde solution in PBS at room temperature. Acetone-paraformaldehyde-fixed cells were permeabilized by using 0.2% Tween 20/PBS, and nonspecific binding sites were blocked by incubation for 1 hr with a 10% solution of heat-inactivated goat serum in 0.2% Tween 20/PBS. To specifically stain neurons, cells were then incubated for 4 hr at room temperature or overnight at 4°C with 1:500 dilutions of anti-MAP-2 (Sigma) or anti-NeuN mAb (Chemicon). Their respective nonspecific isotype antibodies served as controls. After three washes, the cells were incubated in a secondary polyclonal antibody conjugated either to FITC or to horseradish peroxidase. In the case of horseradish peroxidase-coupled polyclonal antibody, diaminobenzidine served as the color substrate developed by incubation in a mixture of 1 mg/ml diaminobenzidine and 0.8% H2O2 at a ratio of 3:1. Cellular nuclei were subsequently stained with 20 μg/ml propidium iodide for 5 min in the dark, and then coverslips were mounted on glass slides. Experiments were replicated at least three times, with triplicate values in each experiment. Statistical significance was determined by using ANOVA followed by a Scheffé or Bonferroni/Dunn post hoc test.
RESULTS AND DISCUSSION
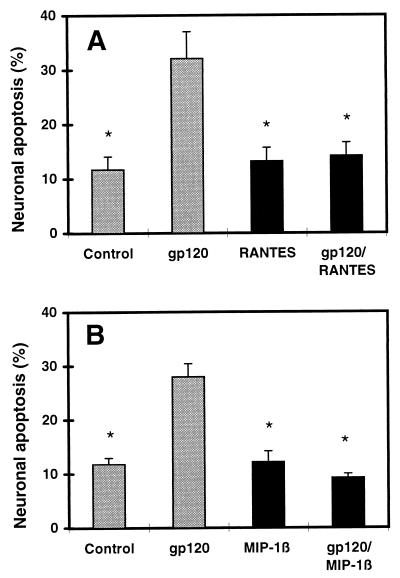
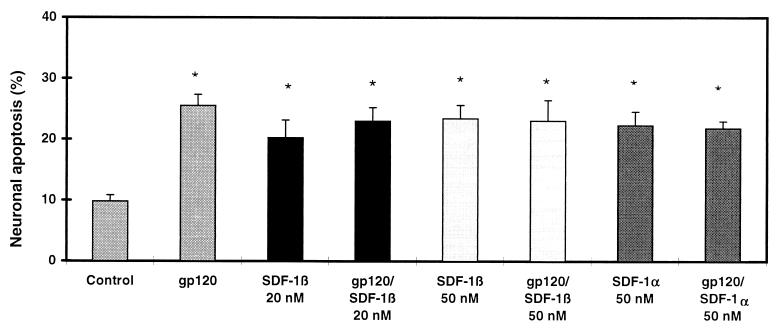
We scored the number of apoptotic cerebrocortical neurons in culture exposed to gp120SF2 by using a combination of propidium iodide staining of permeabilized cells to identify apoptotic nuclei and MAP-2 or NeuN immunostaining to specifically identify neurons (Fig. 1). Additional experiments, by using glial fibrillary acidic protein antibody to identify astrocytes in mixed neuronal/glial cultures or cultures depleted of neurons by prior exposure to NMDA, revealed no significant apoptosis in glial cells after gp120SF2 exposure under our culture conditions (data not shown). The β-chemokines, RANTES and MIP-1β (each at 20 nM), abrogated neuronal apoptosis induced by 200 pM recombinant gp120SF2 (Fig. 2), whereas BSA (0.001% = 144 nM) and the α-chemokines SDF-1α or SDF-1β (20–50 nM) did not protect. In fact, these α-chemokines produced neurotoxicity on their own (≈2-fold increase in neuronal apoptosis compared with control, Fig. 3). MIP-1β and RANTES presumably inhibit the neurotoxic effect of gp120SF2 in an indirect manner, because RANTES binds to the β-chemokine receptors CCR1, CCR3, and CCR5, and MIP-1β binds CCR5 (or a functional rat homologue) (37–39), whereas gp120SF2 (and SDF-1α/β) interact with the α-chemokine receptor CXCR4 (40). Note that although gp120SF2 may also interact to a lesser degree with the β-chemokine receptor CCR5 on some transfected cell lines (41), this has not been shown to occur on primary cells, as used here. In line with these results with gp120SF2, X4 (CXCR4-preferring) virus or dual tropic (X4/R5) virus was recently shown to cause neuronal apoptosis in human cerebrocortical cell cultures (42). Importantly, rodent cerebrocortical cultures are a suitable model system to study these actions of gp120 because these species express CXCR4 homologues that, like the human CXCR4, are capable of mediating HIV-1 infection via gp120 binding (43, 44). Previously, we found in our rodent cultures that gp120-induced neuronal damage was prevented by anti-gp120 antibodies but not by anti-CD4 antibodies, proving the specificity of the effect of gp120 but also implying that CD4 was not necessary for neurotoxicity (4, 30).
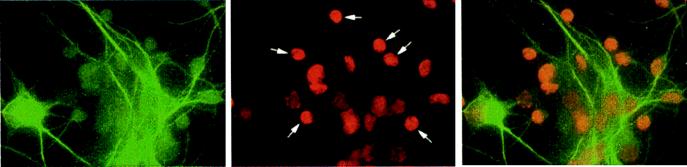
Figure 1.
Immunofluorescence images of neurons labeled with MAP-2 (Left) and propidium iodide (Center) to show apoptosis of neurons (Right, superimposed images) in mixed neuronal/glial cultures after overnight exposure to 200 pM gp120. After propidium iodide labeling, apoptotic neurons appear small, round, and intensely fluorescent (indicated with white arrows in the Center image).
Figure 2.
Protection of rat cerebrocortical neurons from gp120-induced apoptosis by the β-chemokines RANTES and MIP-1β. (A) Neuroprotection by recombinant rat RANTES (20 nM). (B) Neuroprotection by recombinant human MIP-1β (20 nM). Rat cerebrocortical cultures were incubated for 24 hr with or without 200 pM recombinant gp120 and in the presence or absence of each chemokine. After fixation and permeabilization, neurons were identified by immunostaining for MAP-2 or NeuN, and apoptotic cells were assessed by propidium iodide staining. ∗, P < 0.01 compared with value for gp120.
Figure 3.
Neurotoxic effect of SDF-1α and β and lack of protection from gp120-induced apoptosis. Treatment, identification, and analysis of cells as in the legend to Fig. 2. ∗, P < 0.01 compared with value for control but not significantly different from each other.
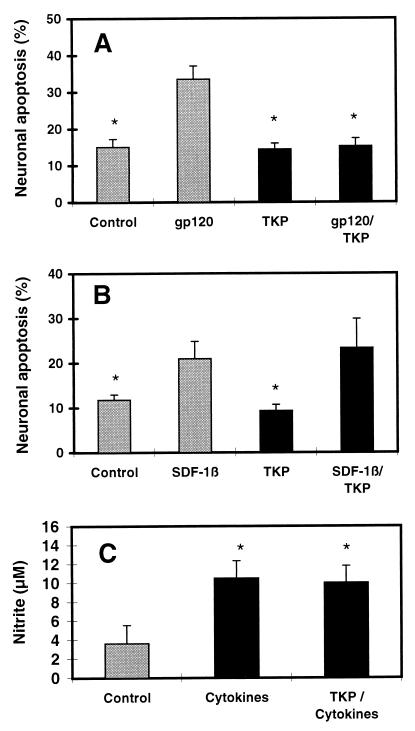
However, these results with RANTES and MIP-1β do not tell us whether the neuroprotective effect of these β-chemokines and, for that matter, the neurotoxic effect of gp120 is mediated by macrophages, astrocytes, neurons, or by simultaneous action on two or all three cell types. To address this query, we used the macrophage inhibitory tripeptide Thr-Lys-Pro (TKP), which has been shown to specifically prevent activation of macrophages/microglia and subsequent release of their toxic factors both in vitro and in vivo, whereas control peptides have no effect (25–28). TKP is comprised of three of the four amino acid residues of tuftsin, a well characterized peptide known to display the opposite effect, i.e., activation of macrophages (45). In our experiments, TKP (50 μM) protected neurons from gp120-induced apoptosis (Fig. 4A), similar to our previous experiments with macrophages depleted from the cultures (18). In contrast, SDF-1β-induced neuronal apoptosis was not abrogated by TKP (Fig. 4B) and also occurred in cultures depleted of macrophages/microglia (data not shown).
Figure 4.
Effects of macrophage-inhibitory peptide TKP on gp120- and SDF-1-induced neuronal apoptosis and on astrocyte activation of immunologic NO synthase. (A) TKP protected neurons from gp120SF2 toxicity. (B) TKP did not protect neurons from SDF-1β toxicity. Experimental conditions and analysis of apoptosis as in the legend to Fig. 2, except TKP (50 μM) was used instead of β-chemokines. ∗, P < 0.01 compared with value for gp120 or SDF-1β. (C) As a control to show that TKP did not prevent astrocyte activation, TKP did not inhibit release of NO from cytokine-stimulated astrocytes in cultures depleted of macrophages/microglia (see Materials and Methods). Astrocytic iNOS was induced by treatment with the cytokines tumor necrosis factor α (200 units/ml), IFN-γ (200 units/ml), and IL-1β (1 ng/ml) for 24 hr in the presence or absence of TKP. “Control” indicates samples without TKP and cytokines. Nitrite levels were monitored in the culture medium as an index of NO release by astrocytes. ∗, P < 0.01 compared with value for control but not significantly different from each other.
Several lines of evidence confirmed prior reports that TKP exerted its effect specifically on macrophages/microglia and not on astrocytes or neurons (25–28). For example, in the absence of macrophages/microglia, TKP did not inhibit NO release by cytokine-activated astrocytes (Fig. 4C), and TKP did not interfere with NMDA-induced neuronal apoptosis (ref. 24; data not shown). These findings indicate that activated macrophages are necessary for gp120SF2- but not SDF-1-induced neuronal apoptosis if the various types and proportion of cells present in the brain are also present in the culture system. This fact does not exclude a direct interaction of gp120 with neuronal or astroglial CXCR4 or other chemokine receptors. But if this interaction occurs, in contrast to the effect of SDF-1, it is apparently not sufficient to trigger neuronal apoptosis in these mixed neuronal/glial cultures.
The number of HIV-1-infected cells in the brain is relatively small, and productively infected cells are exclusively of monocytoid lineage (reviewed in ref. 1). This observation suggests that HIV-1 initiates a neurodegenerative process that entails amplification to produce pronounced central nervous system injury (1). Indeed, in culture systems of both rodent and human brain, HIV-1-infected or gp120-stimulated macrophages and microglia have been found to release neurotoxins that contribute to the neurodegenerative process, at least in part, by excessive stimulation of the NMDA subtype of glutamate receptor (1). The fact that gp120-transgenic mice manifest neuronal damage resembling that found both in rodent cultures and in human brain with HIV-associated dementia indicates that, even in the absence of intact HIV-1, a fragment of the virus is sufficient to trigger important aspects of this amplification cascade in the neurodegenerative process in our in vitro system, which therefore has relevance to in vivo pathogenicity.
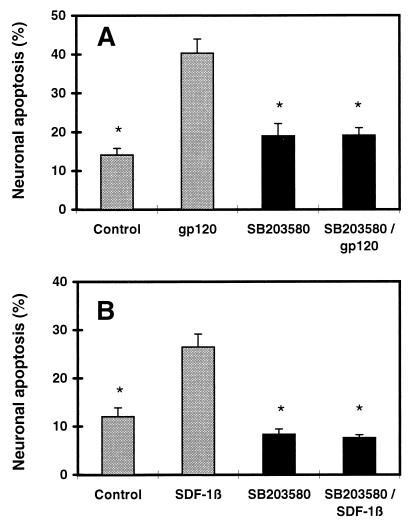
In another series of experiments, we tested a variety of inhibitors of intracellular signaling cascades for their ability to prevent neuronal apoptosis associated with gp120. These inhibitors included PD98059 (2 μM) to inhibit extracellular regulated kinase MAPK, pyrrolidine dithiocarbamate (PDTC, 5 μM) to inhibit NF-κB, and SB203580 (10 μM) to specifically inhibit p38 MAPK (46). Of these, only SB203580 substantially attenuated gp120SF2-induced neuronal apoptosis (Fig. 5A), implicating the p38 MAPK pathway in gp120-activated death signaling. Inhibition of p38 MAPK also ameliorated SDF-1 neurotoxicity (Fig. 5B). This finding indicates that the neurotoxic processes initiated by gp120SF2 and SDF-1 use the common MAPK signaling pathway involving p38. Because SDF-1-induced neurotoxicity occurs in the virtual absence of macrophages/microglia, p38 MAPK must be activated as a stress response in neurons or astrocytes. In fact, from previous work (47, 48), we know that excitotoxic (NMDA) receptor-mediated apoptosis in neurons is mediated, at least in part, by a p38 pathway, and we also know that gp120-induced neuronal damage is prevented by NMDA antagonists (31). Hence, a neuronal p38 pathway perforce must come into play in gp120-induced neurotoxicity. However, we cannot exclude the possibility that gp120 and SDF-1 also activate p38 in macrophages/microglia. In fact, this is likely to occur because activation of p38 MAPK has been reported in activated macrophages/microglia (46). Additionally, immunocytochemical experiments in our culture system have revealed activated (diphosporylated) p38 in both neurons and macrophages (data not shown).
Figure 5.
Inhibition of p38 MAPK reduces gp120- and SDF-1-induced neuronal apoptosis. In the presence or absence of the p38 MAPK inhibitor SB203580 (10 μM), cerebrocortical cultures were incubated for 24 hr with or without 200 pM recombinant gp120SF2 (A) or 20 nM SDF-1β (B). Treatment, identification, and analysis of neurons as in the legend to Fig. 2. ∗, P < 0.01 compared with value for gp120 or SDF-1β.
Taken together, the simplest explanation of our findings with RANTES, MIP-1β, and TKP is that gp120 neurotoxicity depends predominantly on activation of chemokine receptors on macrophages and microglia rather than solely on neurons or astrocytes. In contrast, SDF-1-induced neuronal apoptosis does not require the activation or presence of macrophages/microglia, and therefore the pathophysiologically relevant stimulus for neuronal cell death from this α-chemokine may be transmitted via astrocytes or directly on neurons. Moreover, the fact that the neurotoxic effect of a T cell tropic strain of gp120 (gp120SF2), which has been shown to signal via the α-chemokine receptor CXCR4 (40), can be offset by β-chemokines binding solely to CCR5 (MIP-1β) may indicate that there is a novel pattern of cross-talk between the signaling pathways of various G protein-coupled chemokine receptors. Additionally, although gp120SF2 and SDF-1 differ in the cell type on which they stimulate CXCR4 to induce neuronal apoptosis, both ligands use the p38 MAPK pathway for death signaling. Such signaling cascades may offer new therapeutic targets for interrupting the indirect macrophage pathway to gp120-induced neuronal apoptosis as well as the nonmacrophage-mediated pathway to α-chemokine (SDF-1)-induced neuronal apoptosis.
Acknowledgments
This study was supported by a fellowship award from the Deutsche Forschungsgemeinschaft to M.K. and National Institutes of Health Grants R01 EY09024 and P01 HD29587 to S.A.L.
ABBREVIATIONS
- RANTES
regulated on activation, normal T cell expressed and secreted
- MAPK
mitogen-activated protein kinase
- MAP-2
microtubule-associated protein-2
- NMDA
N-methyl-d-aspartate
- TKP
Thr-Lys-Pro
- MIP
macrophage inflammatory protein
- SDF
stromal cell-derived factor
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Lipton S A, Gendelman H E. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 2.Toggas S M, Masliah E, Rockenstein E M, Rall G F, Abraham C R, Mucke L. Nature (London) 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 3.Brenneman D E, Westbrook G L, Fitzgerald S P, Ennist D L, Elkins K L, Ruff M, Pert C B. Nature (London) 1988;335:639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- 4.Dreyer E B, Kaiser P K, Offermann J T, Lipton S A. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- 5.Müller W E G, Schröder H C, Ushijima H, Dapper J, Bormann J. Eur J Pharmacol. 1992;226:209–214. doi: 10.1016/0922-4106(92)90063-2. [DOI] [PubMed] [Google Scholar]
- 6.Lannuzel A, Lledo P-M, Lamghitnia H O, Vincent J-D, Tardieu M. Eur J Neurosci. 1995;7:2285–2293. doi: 10.1111/j.1460-9568.1995.tb00649.x. [DOI] [PubMed] [Google Scholar]
- 7.Meucci O, Miller R J. J Neurosci. 1996;16:4080–4088. doi: 10.1523/JNEUROSCI.16-13-04080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagetta G, Corasaniti M T, Malorni W, Rainaldi G, Berliocchi L, Finazzi-Agrò A, Nisticò G. NeuroReport. 1996;7:1722–1724. doi: 10.1097/00001756-199607290-00005. [DOI] [PubMed] [Google Scholar]
- 9.Lannuzel A, Barnier J V, Hery C, Van Tan H, Guibert B, Gray F, Vincent J D, Tardieu M. Ann Neurol. 1997;42:847–856. doi: 10.1002/ana.410420605. [DOI] [PubMed] [Google Scholar]
- 10.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. Nature (London) 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 11.Clapham P R. Trends Cell Biol. 1997;7:264–268. doi: 10.1016/S0962-8924(97)01075-1. [DOI] [PubMed] [Google Scholar]
- 12.Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper S C, Hoxie J, Kolson D L, Horuk R. Curr Biol. 1997;7:112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- 13.Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson D L, Horuk R. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- 14.Rottman J B, Ganley K P, Williams K, Wu L, Mackay C R, Ringler D J. Am J Pathol. 1997;151:1341–1350. [PMC free article] [PubMed] [Google Scholar]
- 15.Lavi E, Strizki J M, Ulrich A M, Zhang W, Fu L, Wang Q, O’Connor M, Hoxie J A, González-Scarano F. Am J Pathol. 1997;151:1035–1042. [PMC free article] [PubMed] [Google Scholar]
- 16.Heesen M, Berman M A, Charest A, Housman D, Gerard C, Dorf M E. Immunogenetics. 1998;47:364–370. doi: 10.1007/s002510050371. [DOI] [PubMed] [Google Scholar]
- 17.Meucci O, Fatatis A, Simen A A, Bushnell T J, Gray P W, Miller R J. Proc Natl Acad Sci USA. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipton S A. NeuroReport. 1992;3:913–915. doi: 10.1097/00001756-199210000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Giulian D, Wendt E, Vaca K, Noonan C A. Proc Natl Acad Sci USA. 1993;90:2769–2773. doi: 10.1073/pnas.90.7.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diop A G, Lesort M, Esclaire F, Sindou P, Couratier P, Hugon J. Neurosci Lett. 1994;165:187–190. doi: 10.1016/0304-3940(94)90741-2. [DOI] [PubMed] [Google Scholar]
- 21.Lipton S A. Neurosci Res Commun. 1994;15:31–37. [Google Scholar]
- 22.Flavin M P, Coughline K, Ho L T. Neuroscience. 1997;80:437–448. doi: 10.1016/s0306-4522(97)00078-x. [DOI] [PubMed] [Google Scholar]
- 23.Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O’Brien W A, Verdin E. Nature (London) 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 24.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton S A. Proc Natl Acad Sci USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auriault C, Joseph M, Tartar A, Capron A. FEBS Lett. 1983;153:11–15. doi: 10.1016/0014-5793(83)80109-4. [DOI] [PubMed] [Google Scholar]
- 26.Auriault C, Joseph M, Tartar A, Bout D, Tonnel A B, Capron A. Int J Immunopharmacol. 1985;7:73–79. doi: 10.1016/0192-0561(85)90011-6. [DOI] [PubMed] [Google Scholar]
- 27.Thanos S, Mey J, Wild M. J Neurosci. 1993;13:455–466. doi: 10.1523/JNEUROSCI.13-02-00455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogove A D, Tsirka S E. Curr Biol. 1998;8:19–25. doi: 10.1016/s0960-9822(98)70016-8. [DOI] [PubMed] [Google Scholar]
- 29.Scandella C J, Kilpatrick J, Lidster W, Parker C, Moore J P, Moore G K, Mann K A, Brown P, Coates S, Chapman B, et al. AIDS Res Hum Retroviruses. 1993;9:1233–1244. doi: 10.1089/aid.1993.9.1233. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser P K, Offermann J T, Lipton S A. Neurology. 1990;40:1757–1761. doi: 10.1212/wnl.40.11.1757. [DOI] [PubMed] [Google Scholar]
- 31.Lipton S A, Sucher N J, Kaiser P K, Dreyer E B. Neuron. 1991;7:111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- 32.Lipton S A. Ann Neurol. 1991;30:110–114. doi: 10.1002/ana.410300121. [DOI] [PubMed] [Google Scholar]
- 33.Lipton S A. Nature (London) 1994;367:113–114. doi: 10.1038/367113a0. [DOI] [PubMed] [Google Scholar]
- 34.Lei S Z, Pan Z-H, Aggarwal S K, Chen H-S V, Hartman J, Sucher N J, Lipton S A. Neuron. 1992;8:1087–1099. doi: 10.1016/0896-6273(92)90130-6. [DOI] [PubMed] [Google Scholar]
- 35.Lipton S A, Choi Y-B, Pan Z-H, Lei S Z, Chen H-S V, Sucher N J, Loscalzo J, Singel D J, Stamler J S. Nature (London) 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 36.Chao C C, Hu S, Sheng W S, Bu D, Bukrinsky M I, Peterson P K. Glia. 1996;16:276–284. doi: 10.1002/(SICI)1098-1136(199603)16:3<276::AID-GLIA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 37.Bonini J A, Steiner D F. DNA Cell Biol. 1997;16:1023–1030. doi: 10.1089/dna.1997.16.1023. [DOI] [PubMed] [Google Scholar]
- 38.Luster A D. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 39.Spleiss O, Gourmala N, Boddeke H W, Sauter A, Fiebich B L, Berger M, Gebicke-Haerter P J. J Neurosci Res. 1998;53:16–28. doi: 10.1002/(SICI)1097-4547(19980701)53:1<16::AID-JNR3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 40.Davis C B, Dikic I, Unutmaz D, Hill C M, Arthos J, Siani M A, Thompson D A, Schlessinger J, Littman D R. J Exp Med. 1997;286:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng-Mayer C, Liu R, Landau N R, Stamatatos L. J Virol. 1997;71:1657–1661. doi: 10.1128/jvi.71.2.1657-1661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohagen A, Ghosh S, He J, Huang K, Chen Y, Yuan M, Osathanondh R, Gartner S, Shi B, Shaw G, Gabuzda D. J Virol. 1999;73:897–906. doi: 10.1128/jvi.73.2.897-906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plesoff O, Sol N, Labrosse B, Alizon M. J Virol. 1997;71:3259–3262. doi: 10.1128/jvi.71.4.3259-3262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parolin C B A, Choe H, Farzan M, Kolchinsky P, Heesen M, Ma Q, Gerard C, Palu G, Dorf M E, Springer T, Sodroski J. J Virol. 1998;72:1652–1656. doi: 10.1128/jvi.72.2.1652-1656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Najjar V A. Adv Exp Med Biol. 1979;121:131–147. doi: 10.1007/978-1-4684-3593-1_12. [DOI] [PubMed] [Google Scholar]
- 46.Bhat N R, Zhang P, Lee J C, Hogan E L. J Neurosci. 1998;18:1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawasaki H, Morooka T, Shimohama T, Gotoh Y, Nishida E. J Biol Chem. 1997;272:18518–18521. doi: 10.1074/jbc.272.30.18518. [DOI] [PubMed] [Google Scholar]
- 48.Mukherjee P K, DeCoster M A, Campbell F Z, Davis R J, Bazan N G. J Biol Chem. 1999;274:6493–6498. doi: 10.1074/jbc.274.10.6493. [DOI] [PubMed] [Google Scholar]