Abstract
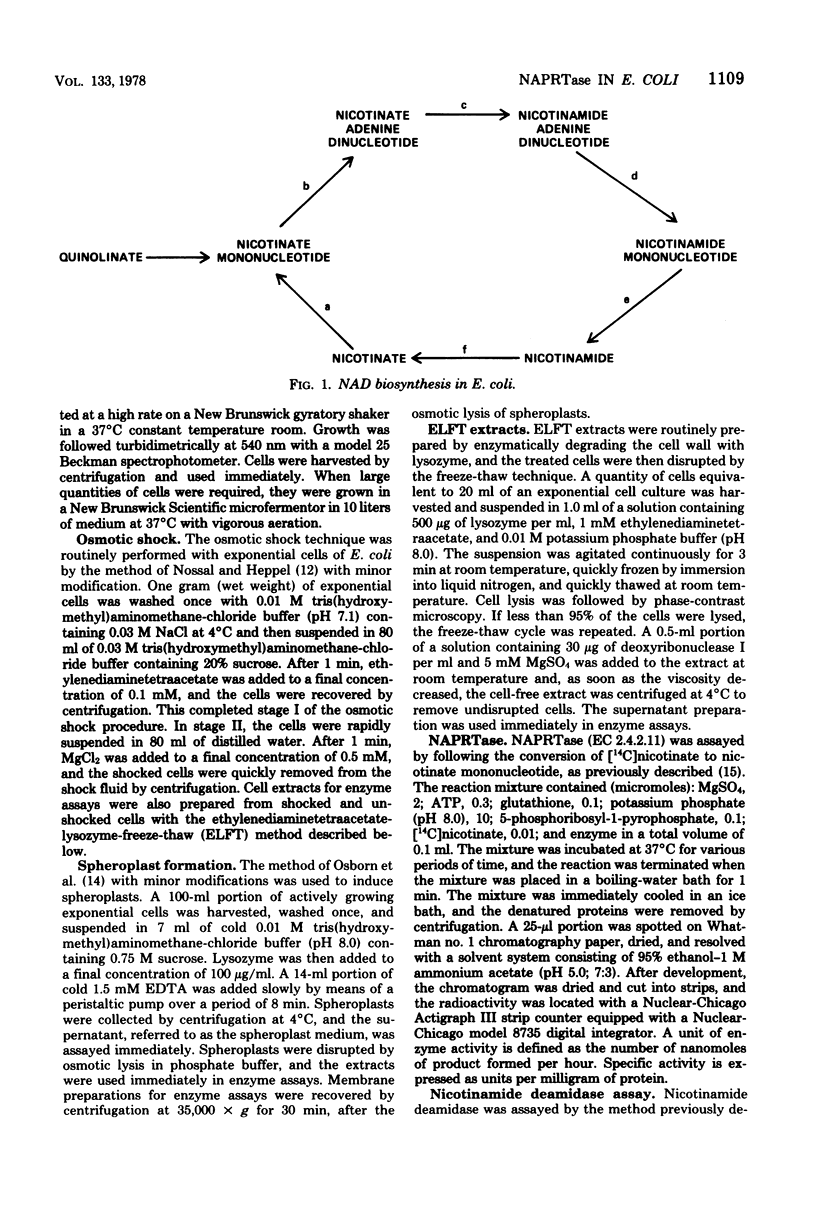
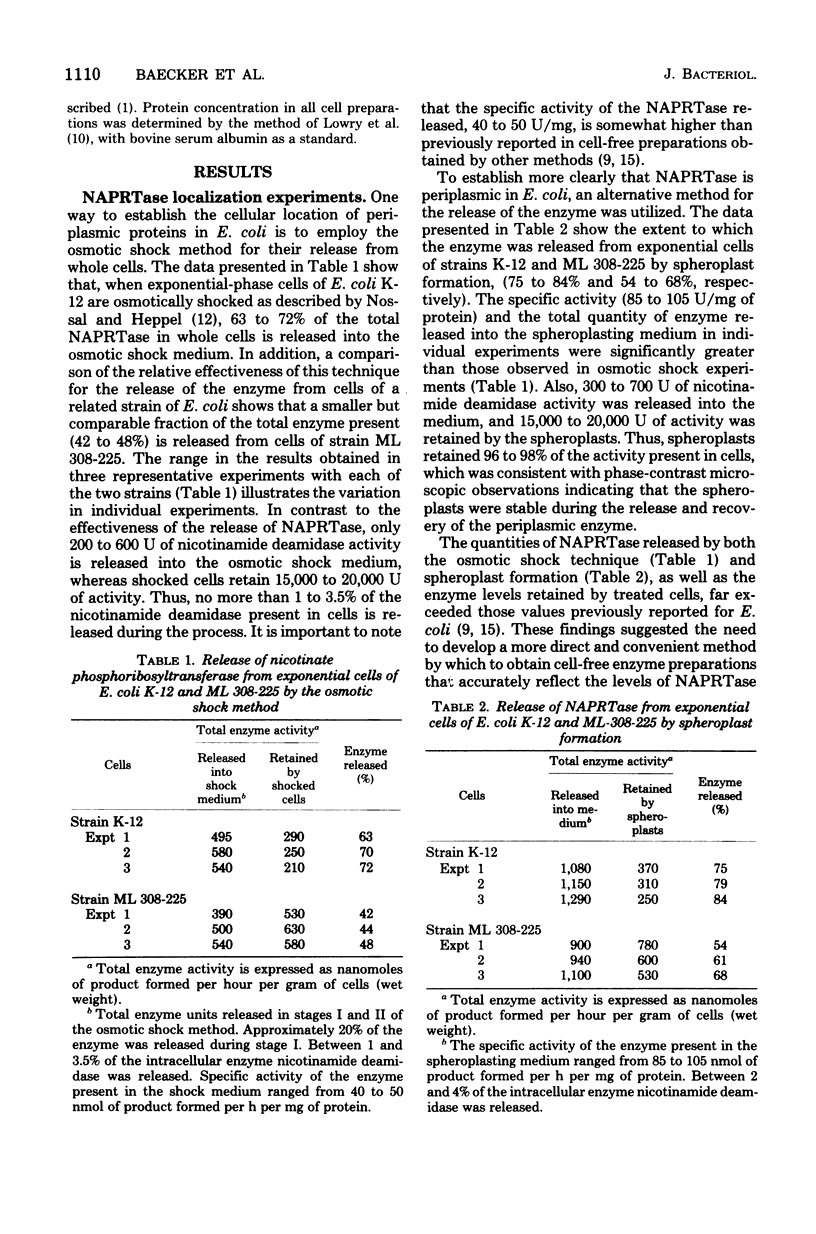
Nicotinate phosphoribosyltransferase (NAPRTase) in Escherichia coli mediates the formation of nicotinate mononucleotide, a direct precursor of nicotinamide adenine dinucleotide (NAD), from nicotinate and 5-phosphoribosyl-1-pyrophosphate. Specifically, NAPRTase contributes to NAD synthesis by utilizing intracellular nicotinate formed from NAD degradation products, which are recycled by NAD cycle enzymes and exogenous nicotinate when it is available. In previous studies, it has been tacitly assumed that almost all NAD cycle enzymes are localized in the cytoplasm of E. coli. The results of this investigation provide evidence that NAPRTase is a periplasmic (extracytoplasmic) enzyme. The osmotic shock of exponential-phase cells of E. coli K-12 and ML 308-225 resulted in the release of 63 to 72% and 42 to 48%, respectively, of the NAPRTase into the shock medium. In addition, when exponential cells of strains K-12 and ML 308-225 were converted into spheroplasts, 75 to 84% and 54 to 68%, respectively, of the enzyme was released into the spheroplast medium. Since previous estimates of the effective levels of NAPRTase present in putative repressed and derepressed E. coli cells appeared to be very low, a more convenient and accurate alternative method for the evaluation of NAPRTase in whole cells was developed. The results show that NAPRTase is subject only to a modest degree of enzyme repression. In addition, no evidence was found for the presence of a protein or low-molecular-weight inhibitor of the enzyme in repressed cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREOLI A. J., IKEDA M., NISHIZUKA Y., HAYAISHI O. Quinolinic acid: a precursor to nicotinamide adenine dinucleotide in Escherichia coli. Biochem Biophys Res Commun. 1963 Jul 18;12:92–97. doi: 10.1016/0006-291x(63)90241-9. [DOI] [PubMed] [Google Scholar]
- Andreoli A. J., Grover T., Gholson R. K., Matney T. S. Evidence for a functional pyridine nucleotide cycle in Escherichia coli. Biochim Biophys Acta. 1969 Dec 30;192(3):539–541. doi: 10.1016/0304-4165(69)90408-5. [DOI] [PubMed] [Google Scholar]
- Andreoli A. J., Okita T. W., Bloom R., Grover T. A. The pyridine nucleotide cycle: presence of a nicotinamide mononucleotide-specific glycohydrolase in Escherichia coli. Biochem Biophys Res Commun. 1972 Oct 6;49(1):264–269. doi: 10.1016/0006-291x(72)90039-3. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. III. The involvement of purine phosphoribosyltransferases in the uptake of adenine and other nucleic acid precursors by intact resting cells. J Biol Chem. 1971 Sep 10;246(17):5312–5320. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McLaren J., Ngo D. T., Olivera B. M. Pyridine nucleotide metabolism in Escherichia coli. 3. Biosynthesis from alternative precursors in vivo. J Biol Chem. 1973 Jul 25;248(14):5144–5149. [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Ogasawara N., Chandler J. L., Gholson R. K., Rosser R. J., Andreoli A. J. Biosynthesis of quinolinic acid in a cell-free system. Biochim Biophys Acta. 1967 Jun 13;141(1):199–201. doi: 10.1016/0304-4165(67)90265-6. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- PARDEE A. B., YATES R. A. Pyrimidine biosynthesis in Escherichia coli. J Biol Chem. 1956 Aug;221(2):743–756. [PubMed] [Google Scholar]
- Saxton R. E., Rocha V., Rosser R. J., Andreoli A. J., Shimoyama M., Kosaka A., Chandler J. L., Gholson R. K. A comparative study of the regulation of nicotinamide-adenine dinucleotide biosynthesis. Biochim Biophys Acta. 1968 Feb 1;156(1):77–84. doi: 10.1016/0304-4165(68)90106-2. [DOI] [PubMed] [Google Scholar]



