Abstract
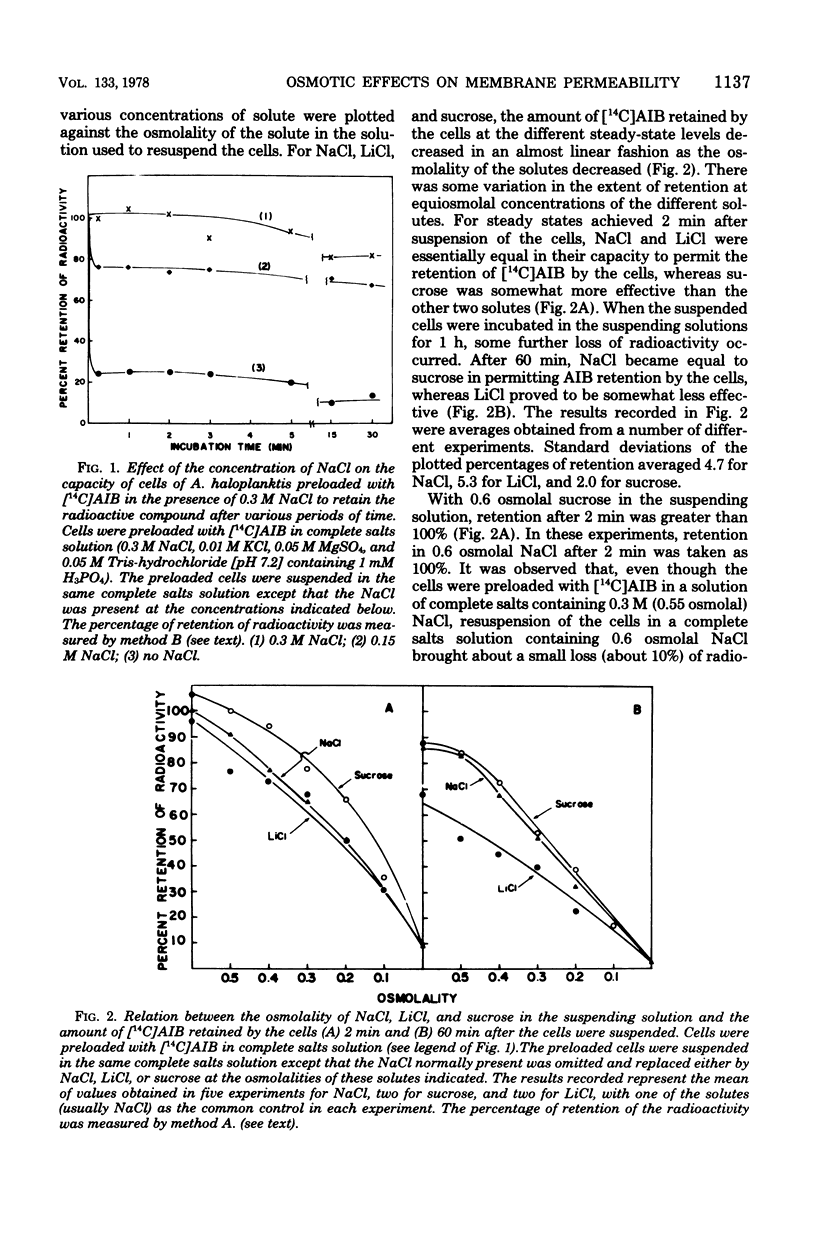
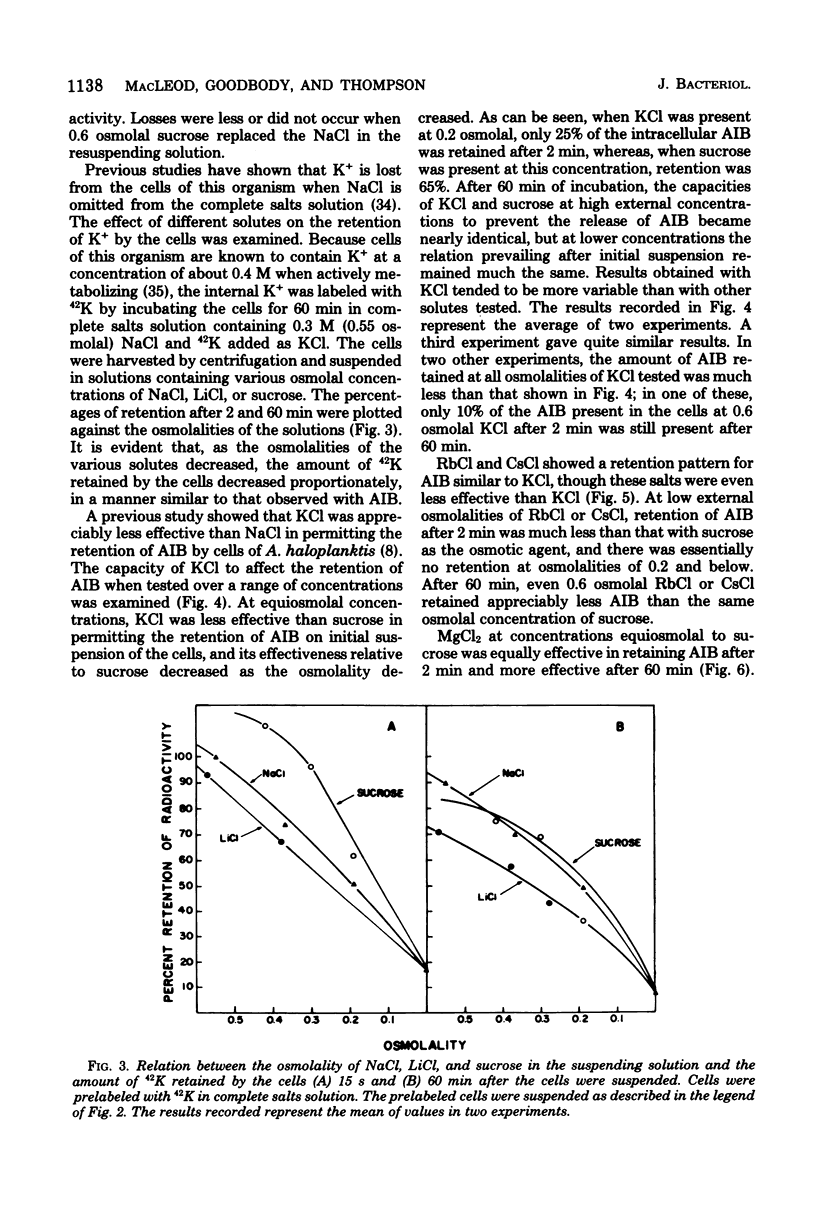
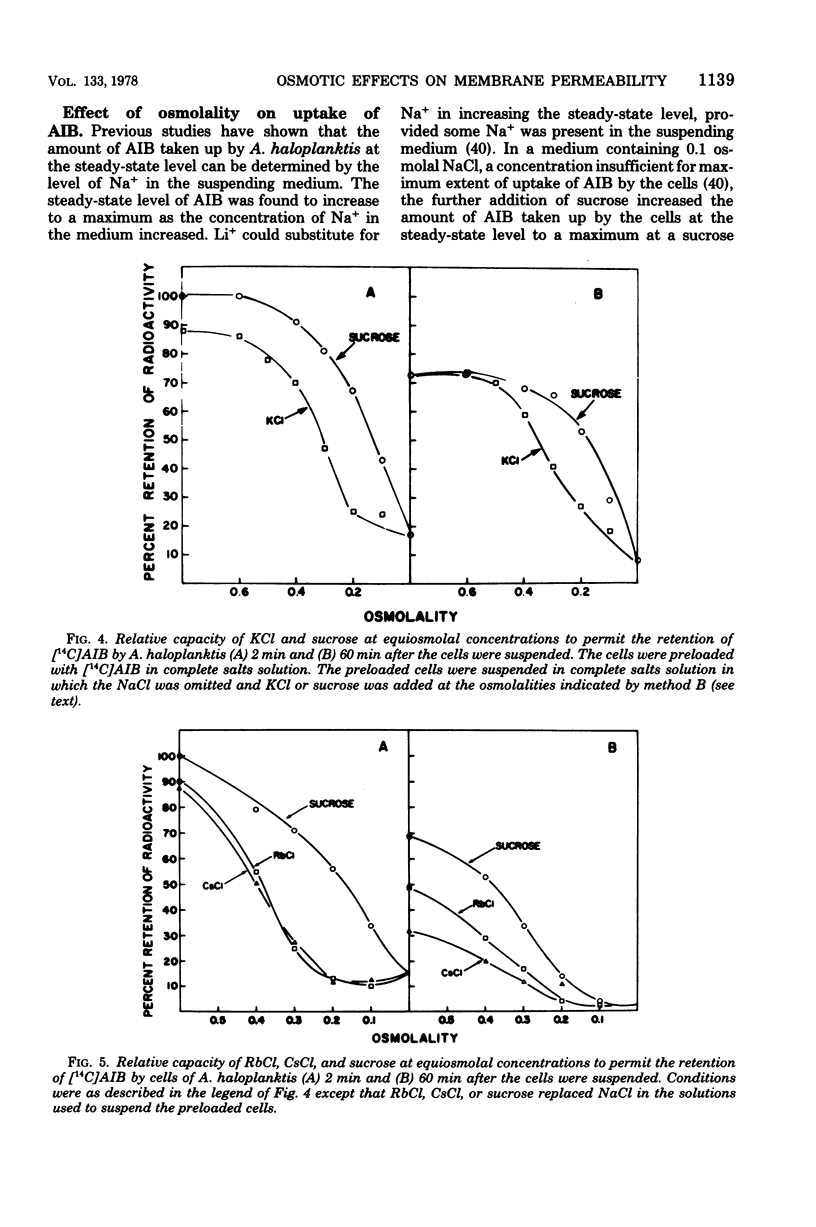
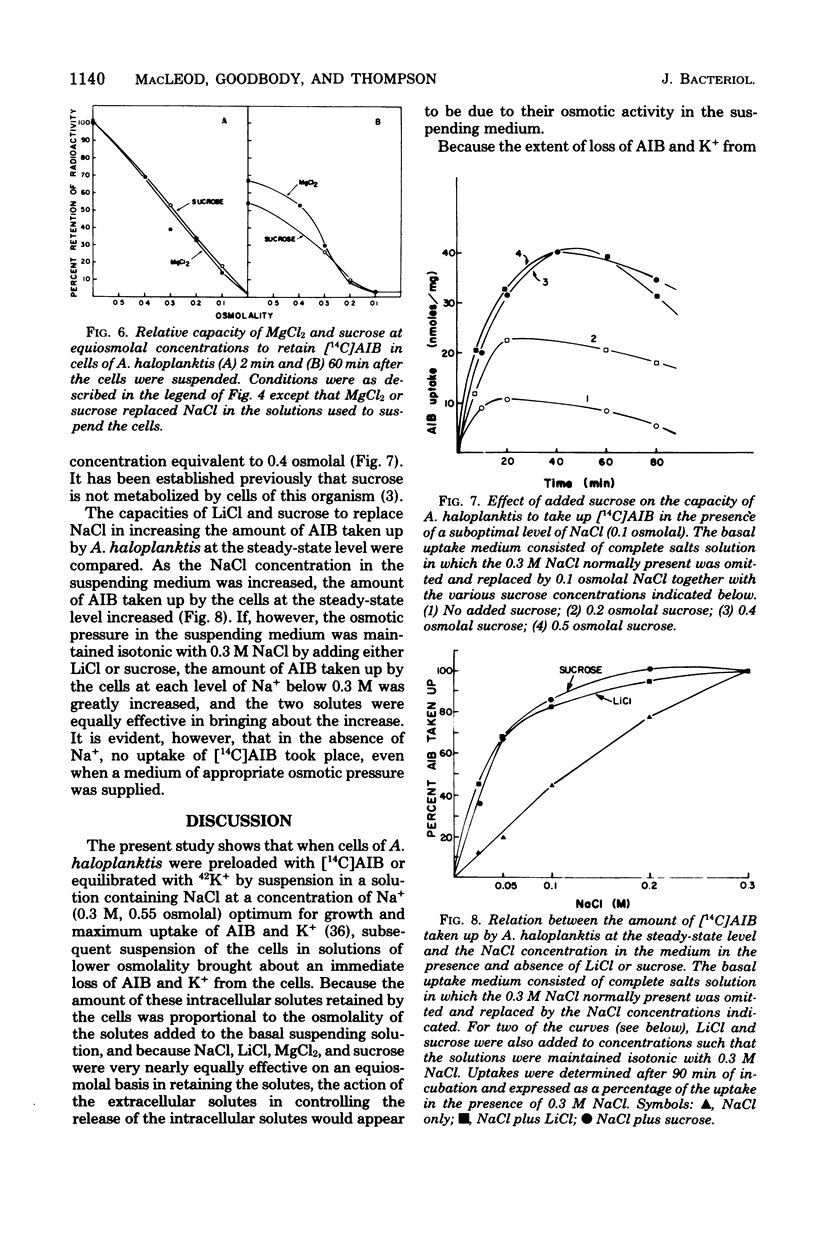
When cells of Alteromonas haloplanktis 214 (ATCC 19855) were preloaded with α-[14C]aminoisobutyric acid or the K+ in the cells was labeled with 42K by incubation in a buffered salt solution containing 0.05 M MgSO4, 0.01 M KCl, and 0.3 M NaCl, the cells retained their radioactivity when resuspended in the same salt solution. When NaCl was omitted from the solution, 80 to 90% of the radioactivity was lost from the cells. Cells suspended at intermediate concentrations of NaCl also lost radioactivity. New steady-state levels of the intracellular solutes were established within 15 s of suspending the cells; the percentage of radioactivity retained at each level decreased proportionately as the osmolality of the NaCl in the suspending solution decreased. With minor variations in effectiveness, MgCl2, LiCl, and sucrose could substitute for NaCl on an equiosmolal basis for the retention of radioactivity by the cells. KCl, RbCl, and CsCl were appreciably less effective as replacements for NaCl, particularly when their osmolalities in the suspending solutions were low. The amount of α-[14C]aminoisobutyric acid taken up by the cells at the steady-state level increased to a maximum as the NaCl concentration in the suspending medium increased to 0.3 M. At suboptimal levels of NaCl, either LiCl or sucrose could substitute for NaCl in increasing the steady-state levels. The results obtained indicate that the porosity of the cytoplasmic membrane of this organism is determined by the difference between the osmotic pressure of the cytoplasm and the suspending medium. The lesser effectiveness of K+, Rb+, and Cs+ than Na+, Li, or Mg2+ in permitting the retention of solutes by the cells is attributed to the greater penetrability of the hydrated ions of the former group through the dilated pores of a stretched cytoplasmic membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIHLER I., CRANE R. K. Studies on the mechanism of intestinal absorption of sugars. V. The influence of several cations and anions on the active transport of sugars, in vitro, by various preparations of hamster small intestine. Biochim Biophys Acta. 1962 May 7;59:78–93. doi: 10.1016/0006-3002(62)90699-6. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., MacLeod R. A. Nutrition and metabolism of marine bacteria. XIV. On the mechanism of lysis of a marine bacterium. Can J Microbiol. 1965 Aug;11(4):677–691. doi: 10.1139/m65-091. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., MacLeod R. A. Penetrability of a marine pseudomonad by inulin, sucrose, and glycerol and its relation to the mechanism of lysis. Can J Microbiol. 1970 Feb;16(2):75–81. doi: 10.1139/m70-014. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corner T. R., Marquis R. E. Why do bacterial protoplasts burst in hypotonic solutions? Biochim Biophys Acta. 1969;183(3):544–558. doi: 10.1016/0005-2736(69)90168-0. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R., MacLeod R. A. A role for inorganic ions in the maintenance of intracellular solute concentrations in a marine pseudomonad. Nature. 1965 May 1;206(983):531–531. doi: 10.1038/206531a0. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R., Matula T. I., MacLeod R. A. Nutrition and metabolism of marine bacteria. XV. Relation of Na+-activated transport to the Na+ requirement of a marine pseudomonad for growth. J Bacteriol. 1966 Jul;92(1):63–71. doi: 10.1128/jb.92.1.63-71.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Rayman M. K., Costerton J. W., MacLeod R. A. Isolation, characterization, and ultrastructure of the peptidoglycan layer of a marine pseudomonad. J Bacteriol. 1972 Feb;109(2):895–905. doi: 10.1128/jb.109.2.895-905.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow J. A., DeVoe U. W., MacLeod R. A. Dissociation in a marine pseudomonad. Can J Microbiol. 1973 Jun;19(6):695–701. doi: 10.1139/m73-113. [DOI] [PubMed] [Google Scholar]
- Halpern Y. S., Barash H., Dover S., Druck K. Sodium and potassium requirements for active transport of glutamate by Escherichia coli K-12. J Bacteriol. 1973 Apr;114(1):53–58. doi: 10.1128/jb.114.1.53-58.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner D. J. Lysis and dissolution of cells and envelopes of an extremely halophilic bacterium. J Bacteriol. 1964 May;87(5):1147–1156. doi: 10.1128/jb.87.5.1147-1156.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E., Corner T. R. Permeability changes associated with osmotic swelling of bacterial protoplasts. J Bacteriol. 1967 Mar;93(3):1177–1178. doi: 10.1128/jb.93.3.1177-1178.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E. Osmotic sensitivity of bacterial protoplasts and the response of their limiting membrane to stretching. Arch Biochem Biophys. 1967 Feb;118(2):323–331. doi: 10.1016/0003-9861(67)90356-6. [DOI] [PubMed] [Google Scholar]
- Morishita H., Takada H. Sparing effect of lithium ion on the specific requirement for sodium ion for growth of Vibrio parahaemolyticus. Can J Microbiol. 1976 Sep;22(9):1263–1268. doi: 10.1139/m76-187. [DOI] [PubMed] [Google Scholar]
- Nelson J. D., Jr, Macleod R. A. Distribution of lipopolysaccharide and the detection of a new subfraction in the cell envelope of a marine pseudomonad. J Bacteriol. 1977 Feb;129(2):1059–1065. doi: 10.1128/jb.129.2.1059-1065.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman M. K., MacLeod R. A. Interaction of Mg-2+ with peptidoglycan and its relation to the prevention of lysis of a marine pseudomonad. J Bacteriol. 1975 May;122(2):650–659. doi: 10.1128/jb.122.2.650-659.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt J. L., Baumann P. Effect of sodium chloride on growth of heterotrophic marine bacteria. Arch Microbiol. 1974 May 20;97(4):329–345. doi: 10.1007/BF00403071. [DOI] [PubMed] [Google Scholar]
- Scholander P. F. Osmotic mechanism and negative pressure. Science. 1967 Apr 7;156(3771):67–69. doi: 10.1126/science.156.3771.67. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Drozdowski J. P., Martin E. L., MacLeod R. A. Kinetics of Naplus-dependent amino acid transport using cells and membrane vesicles of a marine pseudomonad. Can J Microbiol. 1975 Jan;21(1):43–50. doi: 10.1139/m75-006. [DOI] [PubMed] [Google Scholar]
- TAKACS F. P., MATULA T. I., MACLEOD R. A. NUTRITION AND METABOLISM OF MARINE BACTERIA. XIII. INTRACELLULAR CONCENTRATIONS OF SODIUM AND POTASSIUM IONS IN A MARINE PSEUDOMONAD. J Bacteriol. 1964 Mar;87:510–518. doi: 10.1128/jb.87.3.510-518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Costerton J. W., MacLeon R. A. K plus-dependent deplasmolysis of a marine pseudomonad plasmolyzed in a hypotonic solution. J Bacteriol. 1970 Jun;102(3):843–854. doi: 10.1128/jb.102.3.843-854.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Na+ and K+ gradients and alpha-aminoisobutyric acid transport in a marine pseudomonad. J Biol Chem. 1973 Oct 25;248(20):7106–7111. [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Potassium transport and the relationship between intracellular potassium concentration and amino acid uptake by cells of a marine pseudomonad. J Bacteriol. 1974 Nov;120(2):598–603. doi: 10.1128/jb.120.2.598-603.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapis A., Kepes A. Transient breakdown of the permeability barrier of the membrane of Escherichia coli upon hypoosmotic shock. Biochim Biophys Acta. 1977 Aug 15;469(1):1–12. doi: 10.1016/0005-2736(77)90320-0. [DOI] [PubMed] [Google Scholar]
- Unemoto T., Tsuruoka T., Hayashi M. Role of Na + and K + in preventing lysis of a slightly halophilic Vibrio alginolyticus. Can J Microbiol. 1973 May;19(5):563–571. doi: 10.1139/m73-093. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Thompson J., MacLeod R. A. Nutrition and metabolism of marine bacteria. XVII. Ion-dependent retention of alpha-aminoisobutyric acid and its relation to Na+ dependent transport in a marine pseudomonad. J Biol Chem. 1969 Feb 10;244(3):1016–1025. [PubMed] [Google Scholar]



