Abstract
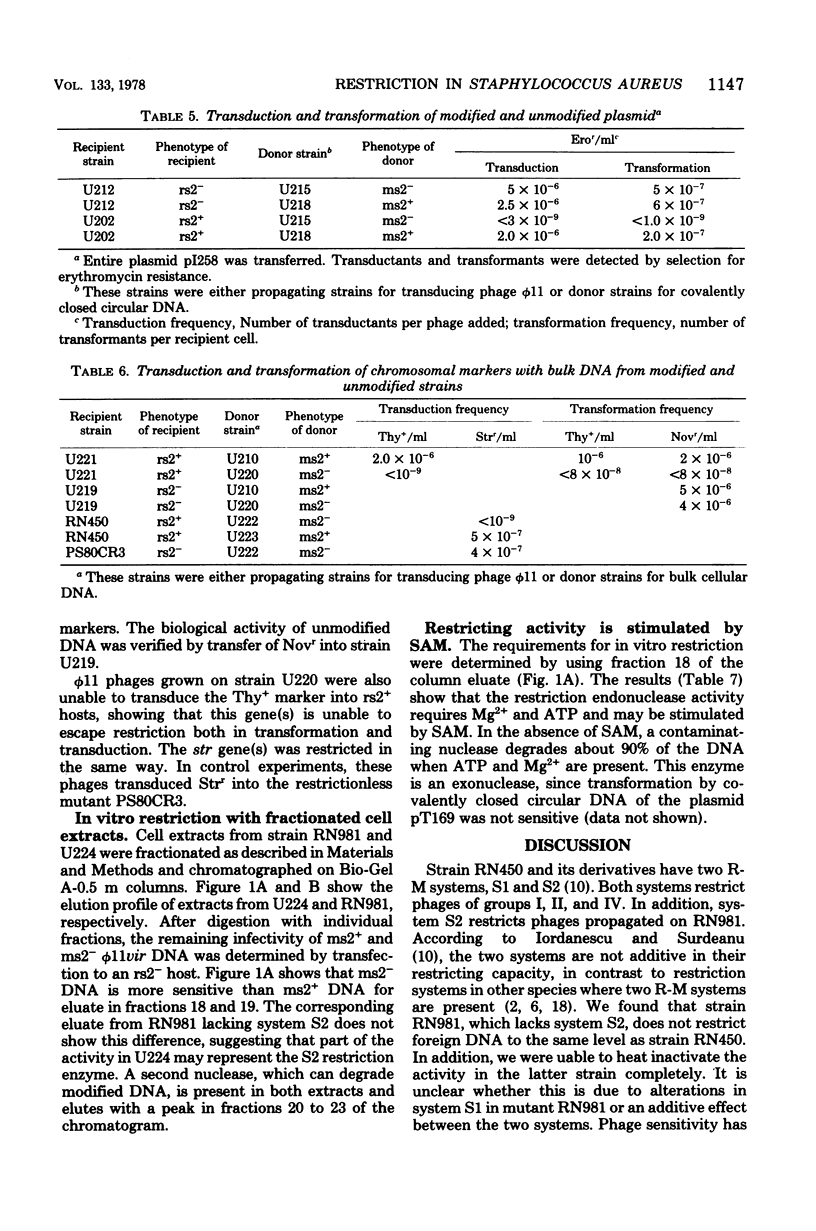
Two restriction-modification systems, S1 and S2, are present in Staphylococcus aureus RN450 (S. Iordanescu and M. Surdeanu, J. Gen. Microbiol., 96:277-281, 1976). System S2 affects phage multiplication after both infection and transfection. Unmodified plasmid and chromosomal DNAs are also not expressed following transduction and transformation into a restrictive host. Restricted phages are, however, capable of conferring phage-mediated competence, although the state of competence does not affect the restriction-modification system. The restricting activity of system S2 is inactivated by heat treatment of the cells. An enzymatic activity that restricts unmodified phage DNA in the presence of ATP, Mg2+, and S-adenosylmethionine was recovered from cell-free extracts of a strain RN450 derivative.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHESHOV E. A., JEVONS M. P. The effect of heat on the ability of a host strain to support the growth of a Staphylococcus phage. J Gen Microbiol. 1963 Apr;31:97–107. doi: 10.1099/00221287-31-1-97. [DOI] [PubMed] [Google Scholar]
- Arber W., Linn S. DNA modification and restriction. Annu Rev Biochem. 1969;38:467–500. doi: 10.1146/annurev.bi.38.070169.002343. [DOI] [PubMed] [Google Scholar]
- Arber W., Wauters-Willems D. Host specificity of DNA produced by Escherichia coli. XII. The two restriction and modification systems of strain 15T-. Mol Gen Genet. 1970;108(3):203–217. doi: 10.1007/BF00283350. [DOI] [PubMed] [Google Scholar]
- Boyer H. W. DNA restriction and modification mechanisms in bacteria. Annu Rev Microbiol. 1971;25:153–176. doi: 10.1146/annurev.mi.25.100171.001101. [DOI] [PubMed] [Google Scholar]
- Boyer H. W. Restriction and modification of DNA: enzymes and substrates. Introductory remarks. Fed Proc. 1974 May;33(5):1125–1127. [PubMed] [Google Scholar]
- Colson C., Van Pel A. DNA restriction and modification systems in Salmonella. I. SA and SB, two Salmonella typhimurium systems determined by genes with a chromosomal location comparable to that of the Escherichia coli hsd genes. Mol Gen Genet. 1974 Apr 3;129(4):325–337. doi: 10.1007/BF00265696. [DOI] [PubMed] [Google Scholar]
- DUSSOIX D., ARBER W. HOST SPECIFICITY OF DNA PRODUCED BY ESCHERICHIA COLI. IV. HOST SPECIFICITY OF INFECTIOUS DNA FROM BACTERIOPHAGE LAMBDA. J Mol Biol. 1965 Feb;11:238–246. doi: 10.1016/s0022-2836(65)80054-7. [DOI] [PubMed] [Google Scholar]
- Gromkova R., Bendler J., Goodgal S. Restriction and modification of bacteriophage S2 in Haemophilus influenzae. J Bacteriol. 1973 Jun;114(3):1151–1157. doi: 10.1128/jb.114.3.1151-1157.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubacek J., Glover S. W. Complementation analysis of temperature-sensitive host specificity mutations in Escherichia coli. J Mol Biol. 1970 May 28;50(1):111–127. doi: 10.1016/0022-2836(70)90108-7. [DOI] [PubMed] [Google Scholar]
- Iordanescu S., Surdeanu M. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J Gen Microbiol. 1976 Oct;96(2):277–281. doi: 10.1099/00221287-96-2-277. [DOI] [PubMed] [Google Scholar]
- Lindberg M., Sjöström J. E., Johansson T. Transformation of chromosomal and plasmid characters in Staphylococcus aureus. J Bacteriol. 1972 Feb;109(2):844–847. doi: 10.1128/jb.109.2.844-847.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn S., Lautenberger J. A., Eskin B., Lackey D. Host-controlled restriction and modification enzymes of Escherichia coli B. Fed Proc. 1974 May;33(5):1128–1134. [PubMed] [Google Scholar]
- Meselson M., Yuan R., Heywood J. Restriction and modification of DNA. Annu Rev Biochem. 1972;41:447–466. doi: 10.1146/annurev.bi.41.070172.002311. [DOI] [PubMed] [Google Scholar]
- Nathans D., Smith H. O. Restriction endonucleases in the analysis and restructuring of dna molecules. Annu Rev Biochem. 1975;44:273–293. doi: 10.1146/annurev.bi.44.070175.001421. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Piekarowicz A., Glover S. W. Host specificity of DNA in Haemophilus influenzae: the two restriction and modification systems in strain Ra. Mol Gen Genet. 1972;116(1):11–25. doi: 10.1007/BF00334255. [DOI] [PubMed] [Google Scholar]
- RALSTON D. J., BAER B. S. A NEW PROPERTY OF PHAGE GROUP II STAPHYLOCOCCUS AUREUS STRAINS: HOST RESTRICTION OF PHAGE K14. J Gen Microbiol. 1964 Jul;36:1–16. doi: 10.1099/00221287-36-1-1. [DOI] [PubMed] [Google Scholar]
- ROUNTREE P. M. Variations in a related series of staphylococcal bacteriophages. J Gen Microbiol. 1956 Oct;15(2):266–279. doi: 10.1099/00221287-15-2-266. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction endonucleases. CRC Crit Rev Biochem. 1976 Nov;4(2):123–164. doi: 10.3109/10409237609105456. [DOI] [PubMed] [Google Scholar]
- Roulland-Dussoix D., Boyer H. W. The Escherichia coli B restriction endonuclease. Biochim Biophys Acta. 1969 Nov 19;195(1):219–229. doi: 10.1016/0005-2787(69)90618-2. [DOI] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Competence for transfection in Staphylococcus aureus. J Bacteriol. 1973 Feb;113(2):576–585. doi: 10.1128/jb.113.2.576-585.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Transfection of Staphylococcus aureus with bacteriophage deoxyribonucleic acid. J Bacteriol. 1972 Jan;109(1):285–291. doi: 10.1128/jb.109.1.285-291.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Löfdahl S., Philipson L. Transformation reveals a chromosomal locus of the gene(s) for methicillin resistance in Staphylococcus aureus. J Bacteriol. 1975 Sep;123(3):905–915. doi: 10.1128/jb.123.3.905-915.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Philipson L. Role of the phi 11 phage genome in competence of Staphylococcus aureus. J Bacteriol. 1974 Jul;119(1):19–32. doi: 10.1128/jb.119.1.19-32.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobberingh E. E., Winkler K. C. Restriction-deficient mutants of Staphylococcus aureus. J Gen Microbiol. 1977 Apr;99(2):359–367. doi: 10.1099/00221287-99-2-359. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., Monfoort C. H., Schiphof R., Stobberingh E. E. A restriction endonuclease from Staphylococcus aureus. Nucleic Acids Res. 1976 Nov;3(11):3193–3202. doi: 10.1093/nar/3.11.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzi M. Physiology of bacteriophage lambda in the restrictive host. J Mol Biol. 1968 May 28;34(1):165–173. doi: 10.1016/0022-2836(68)90242-8. [DOI] [PubMed] [Google Scholar]
- Trautner T. A., Pawlek B., Bron S., Anagnostopoulos C. Restriction and modification in B. subtilis. Biological aspects. Mol Gen Genet. 1974;131(3):181–191. doi: 10.1007/BF00267958. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. E. O., RIPPON J. E. Bacteriophage typing of Staphylococcus aureus. J Hyg (Lond) 1952 Sep;50(3):320–353. doi: 10.1017/s002217240001963x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman L., Goering R. V., Novick R. P. Genetic control of chromosomal and plasmid recombination in Staphylococcus aureus. Genetics. 1974 Apr;76(4):681–702. doi: 10.1093/genetics/76.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]



