Abstract
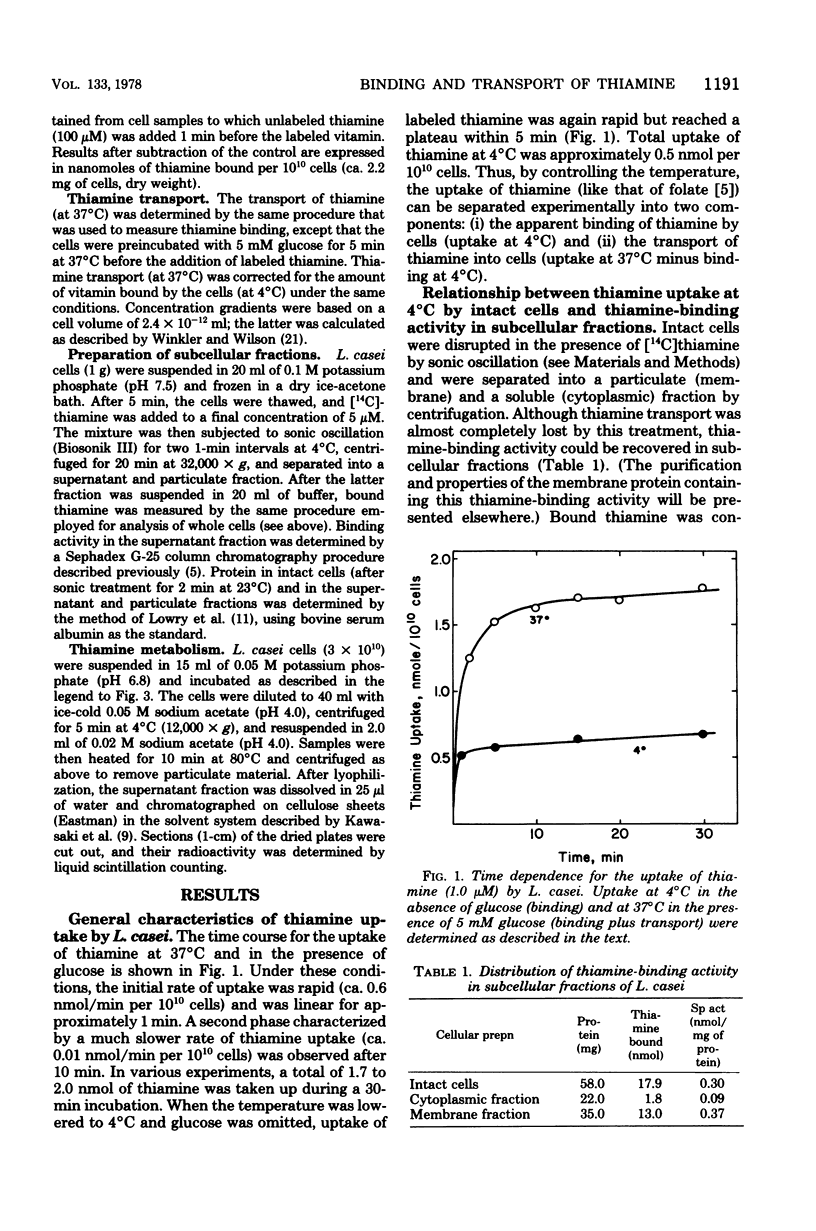
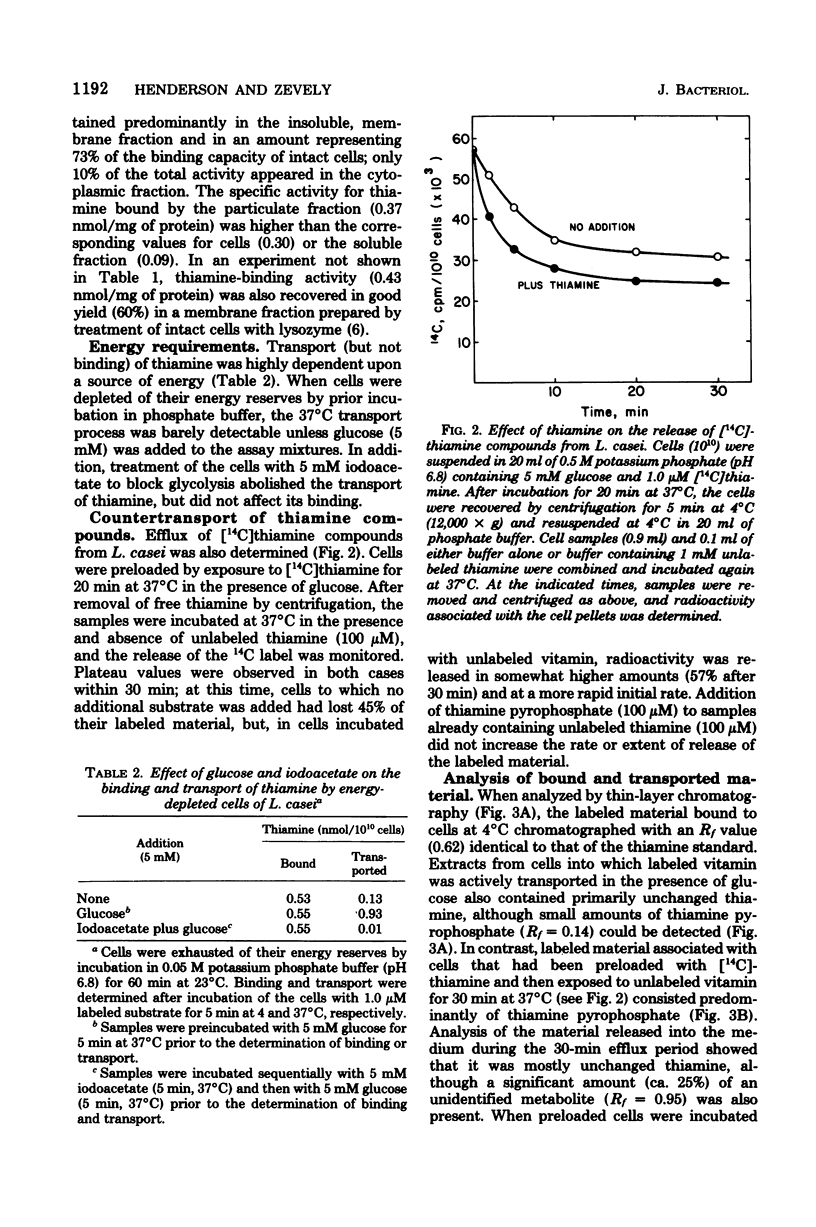
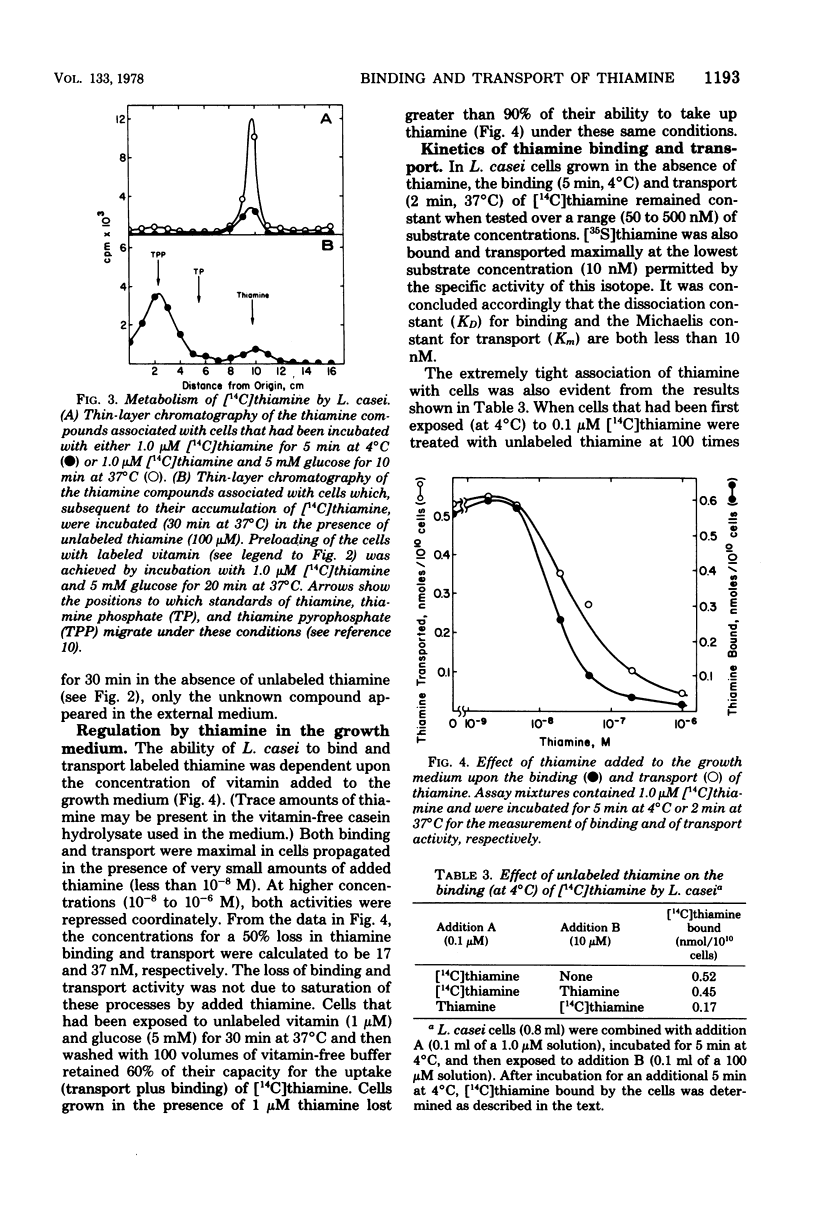
The relationship between thiamine transport and a membrane-associated thiamine-binding activity has been investigated in Lactobacillus casei. Thiamine transport proceeds via a system whose general properties are typical of active uptake processes; entry of the vitamin into the cells requires energy, is temperature dependent, exhibits saturation kinetics, and is inhibited by substrate analogs. A considerable concentration gradient of unchanged thiamine can be achieved by the system, although the vitamin is slowly metabolized to thiamine pyrophosphate. Consistent with these results, L. casei also contains a high-affinity, thiamine-binding component which could be measured by incubation of intact cells with labeled substrate at 4 degrees C (conditions under which transport is negligible). Binding was insensitive to iodoacetate, occurred at a level (0.5 nmol per 10(10) cells) nearly 20-fold higher than could be accounted for by facilitated diffusion, and was found to reside in a component of the cell membrane. Participation of this binder in thiamine transport is supported by the observations that the processes of binding and transport showed similarities in their (i) regulation by the concentration of thiamine in the growth medium, (ii) binding affinities for thiamine, and (iii) susceptibility to inhibition by thiamine analogs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper B. A. Studies of (3H)folic acid uptake by Lactobacillus casei. Biochim Biophys Acta. 1970 Apr 14;208(1):99–109. doi: 10.1016/0304-4165(70)90052-8. [DOI] [PubMed] [Google Scholar]
- Gordon A. S., Lombardi F. J., Kaback H. R. Solubilization and partial purification of amino acid-specific components of the D-lactate dehydrogenase-coupled amino acid-transport systems (E. coli-cell membranes-sephadex-detergent-solubilized-vesicles). Proc Natl Acad Sci U S A. 1972 Feb;69(2):358–362. doi: 10.1073/pnas.69.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G. B., Huennekens F. M. Transport of folate compounds into Lactobacillus Casei. Arch Biochem Biophys. 1974 Oct;164(2):722–728. doi: 10.1016/0003-9861(74)90085-x. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Huennekens F. M. Folate transport in Lactobacillus casei: solubilization and general properties of the binding protein. Biochem Biophys Res Commun. 1976 Feb 9;68(3):712–717. doi: 10.1016/0006-291x(76)91203-1. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Huennekens F. M. Purification and properties of a membrane-associated, folate-binding protein from Lactobacillus casei. J Biol Chem. 1977 Jun 10;252(11):3760–3765. [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Kadner R. J., Huennekens F. M. The folate and thiamine transport proteins of Lactobacillus casei. J Supramol Struct. 1977;6(2):239–247. doi: 10.1002/jss.400060209. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Miyata I., Esaki K., Nose Y. Thiamine uptake in Escherichia coli. I. General properties of thiamine uptake system in Escherichia coli. Arch Biochem Biophys. 1969 Apr;131(1):223–230. doi: 10.1016/0003-9861(69)90125-8. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Yamada K. The uptake system of free thiamine in mutants of Escherichia coli. Biochem Biophys Res Commun. 1972 Apr 28;47(2):465–471. doi: 10.1016/0006-291x(72)90737-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsuura A., Iwashima A., Nose Y. Purification of thiamine-binding protein from Escherichia coli. Biochem Biophys Res Commun. 1973 Mar 5;51(1):241–246. doi: 10.1016/0006-291x(73)90534-2. [DOI] [PubMed] [Google Scholar]
- Morishita T., Fukada T., Shirota M., Yura T. Genetic basis of nutritional requirements in Lactobacillus casei. J Bacteriol. 1974 Dec;120(3):1078–1084. doi: 10.1128/jb.120.3.1078-1084.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neujahr H. Y. Transport of B-vitamins in microorganisms. 3. Chromatographic studies on the radioactivity extracted from non-proliferating cells of Lactobacillus fermenti after exposure to labelled thiamine. Acta Chem Scand. 1966;20(3):786–798. doi: 10.3891/acta.chem.scand.20-0786. [DOI] [PubMed] [Google Scholar]
- Neujahr H. Y. Transport of B-vitamins in microorganisms. II. Factors affecting the uptake of labelled thiamine by nonproliferating cells of Lactobacillus fermenti. Acta Chem Scand. 1966;20(3):771–785. doi: 10.3891/acta.chem.scand.20-0771. [DOI] [PubMed] [Google Scholar]
- Neujahr H. Y. Transport of B-vitamins in microorganisms. VI. The non-specificity of the effect of exogeneous ATP on the uptake of labelled thiamine by non-proliferating thiamine deficient cells of L. fermenti. A reappraisal. Acta Chem Scand. 1966;20(3):894–895. doi: 10.3891/acta.chem.scand.20-0894. [DOI] [PubMed] [Google Scholar]
- Shane B., Stokstad E. L. Transport and utilization of methyltetrahydrofolates by Lactobacillus casei. J Biol Chem. 1976 Jun 10;251(11):3405–3410. [PubMed] [Google Scholar]



