Abstract
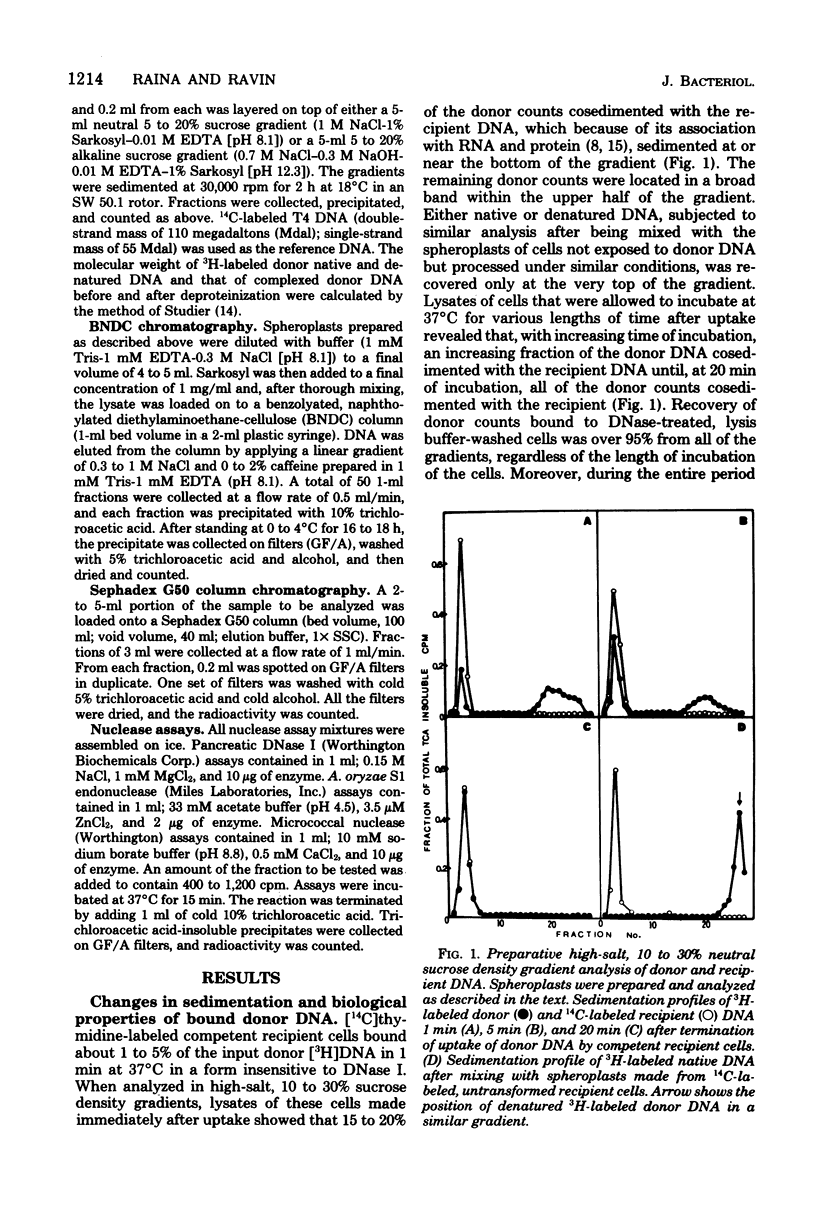
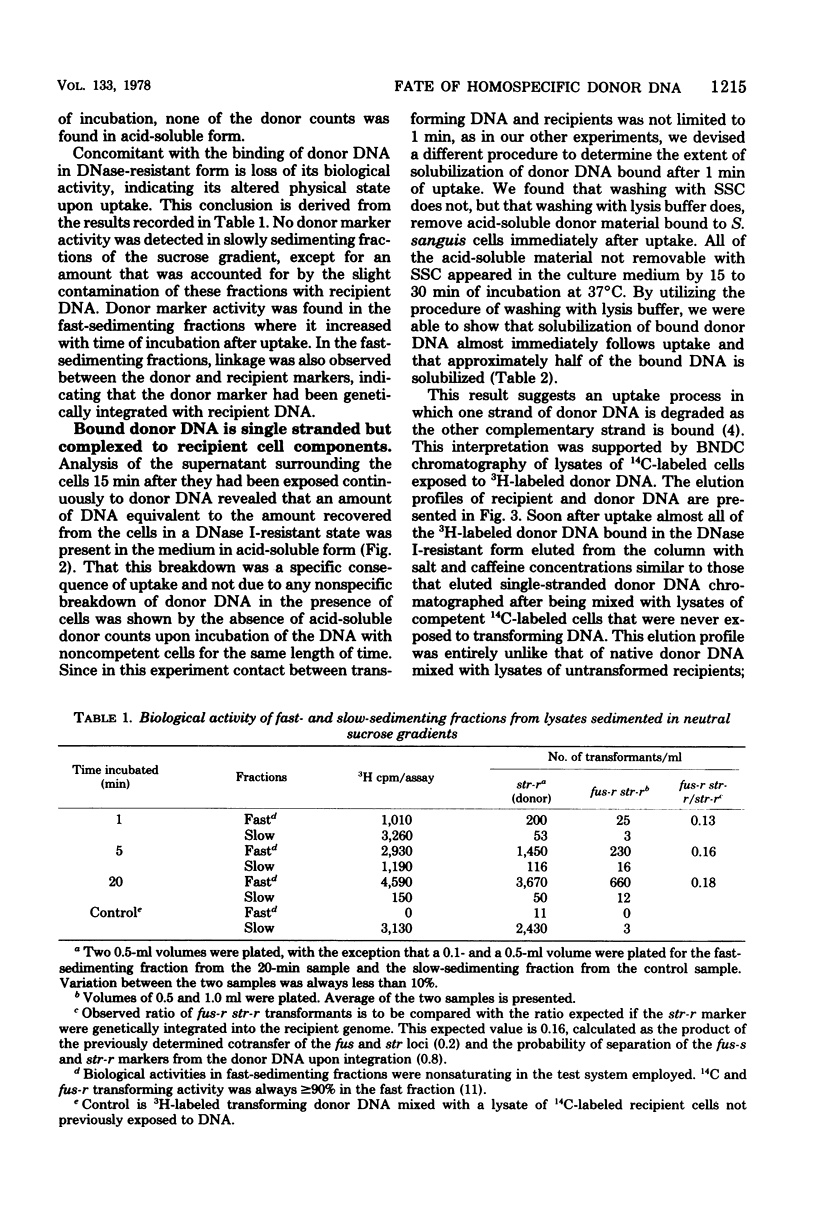
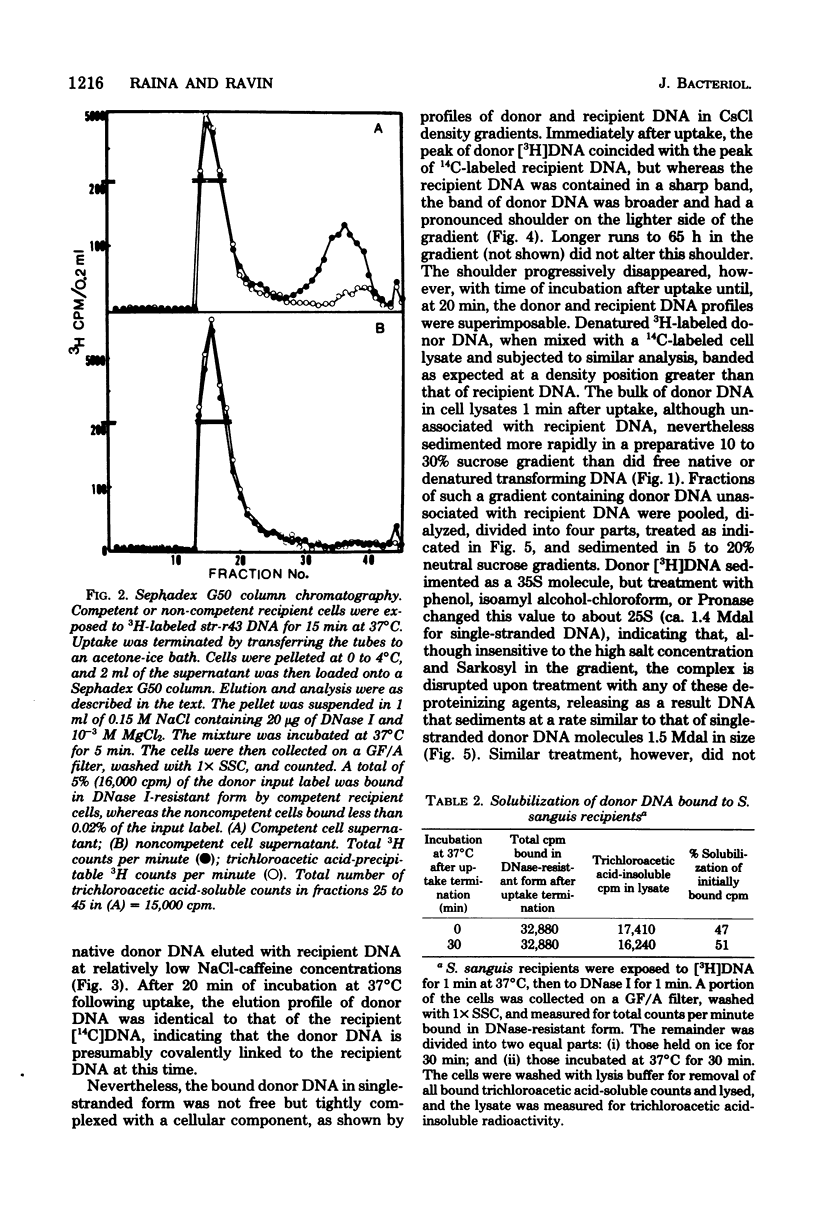
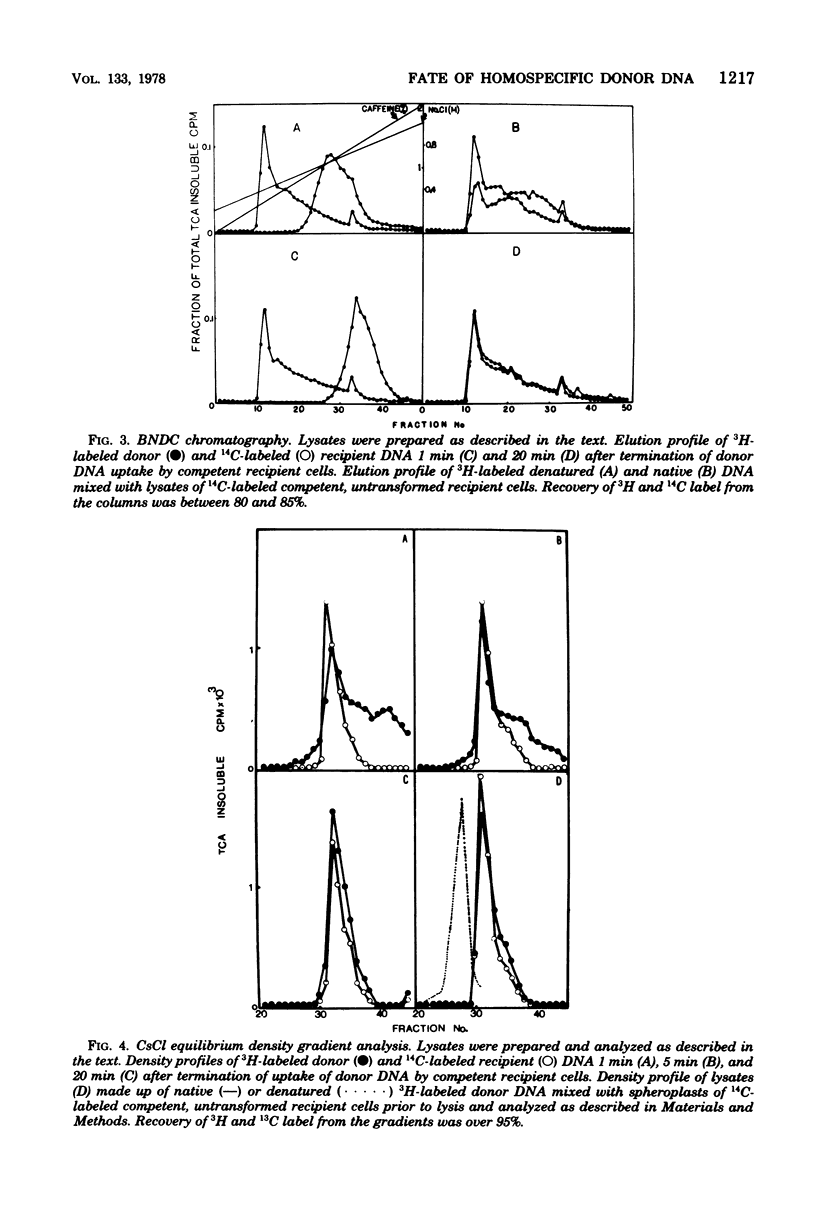
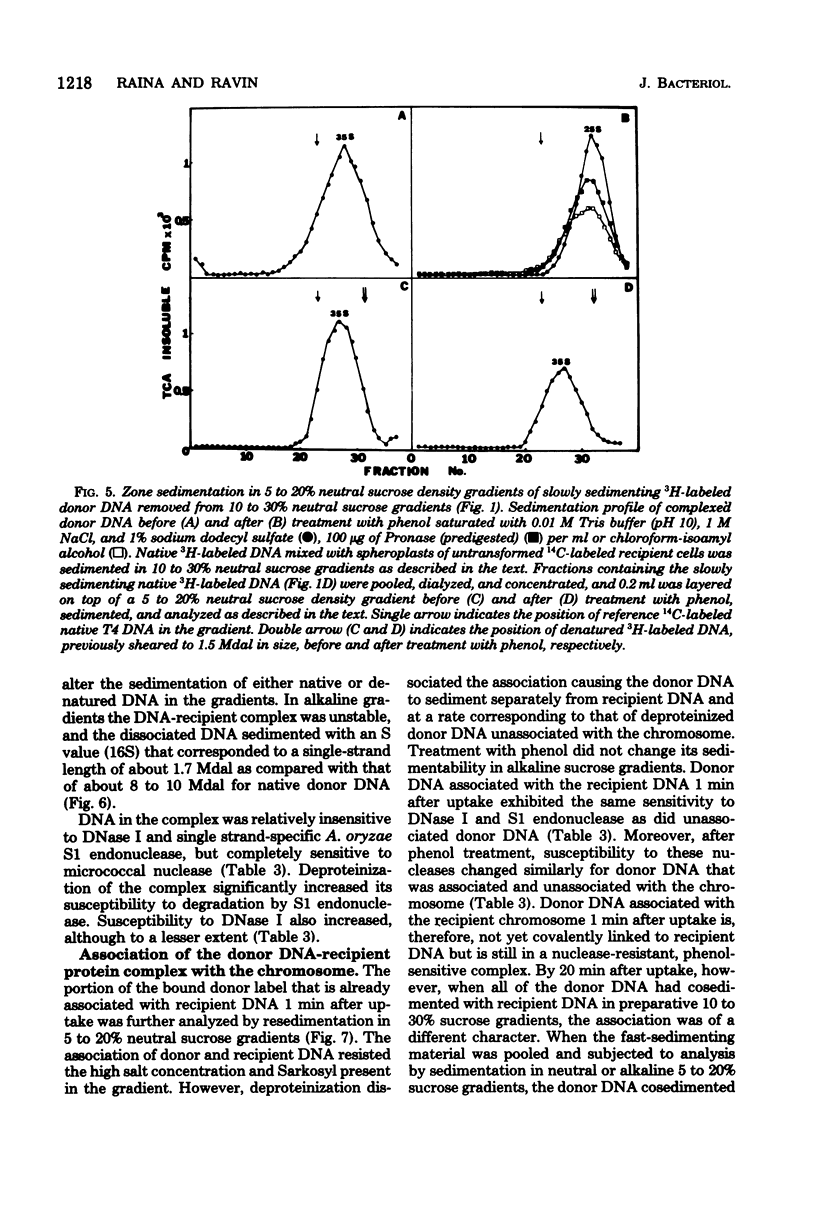
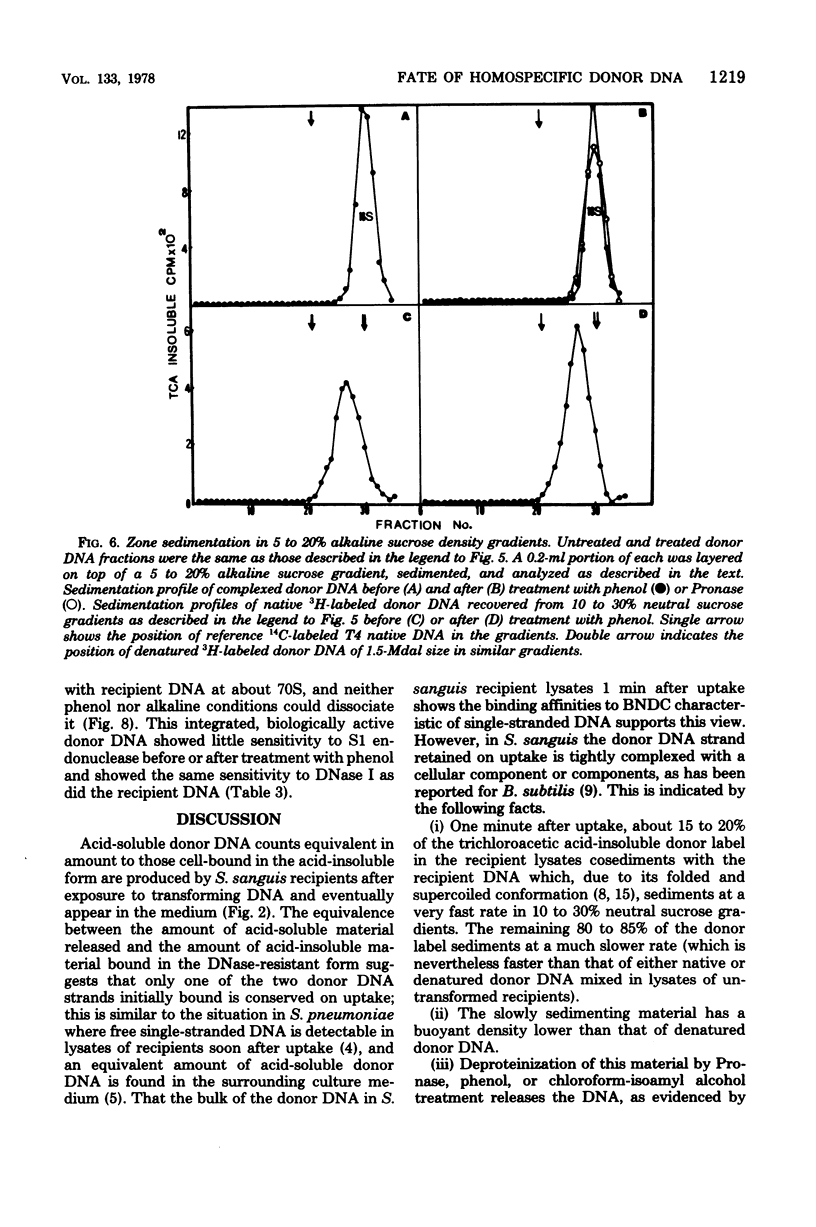
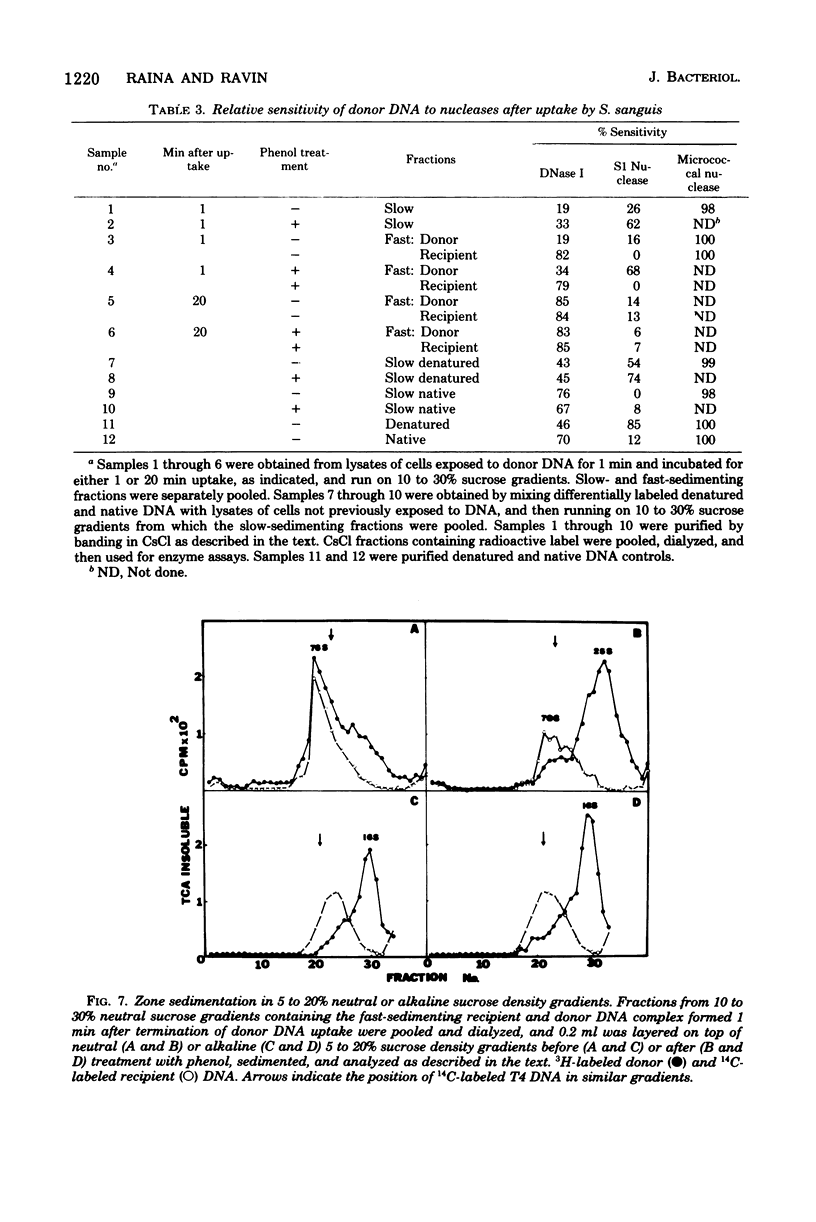
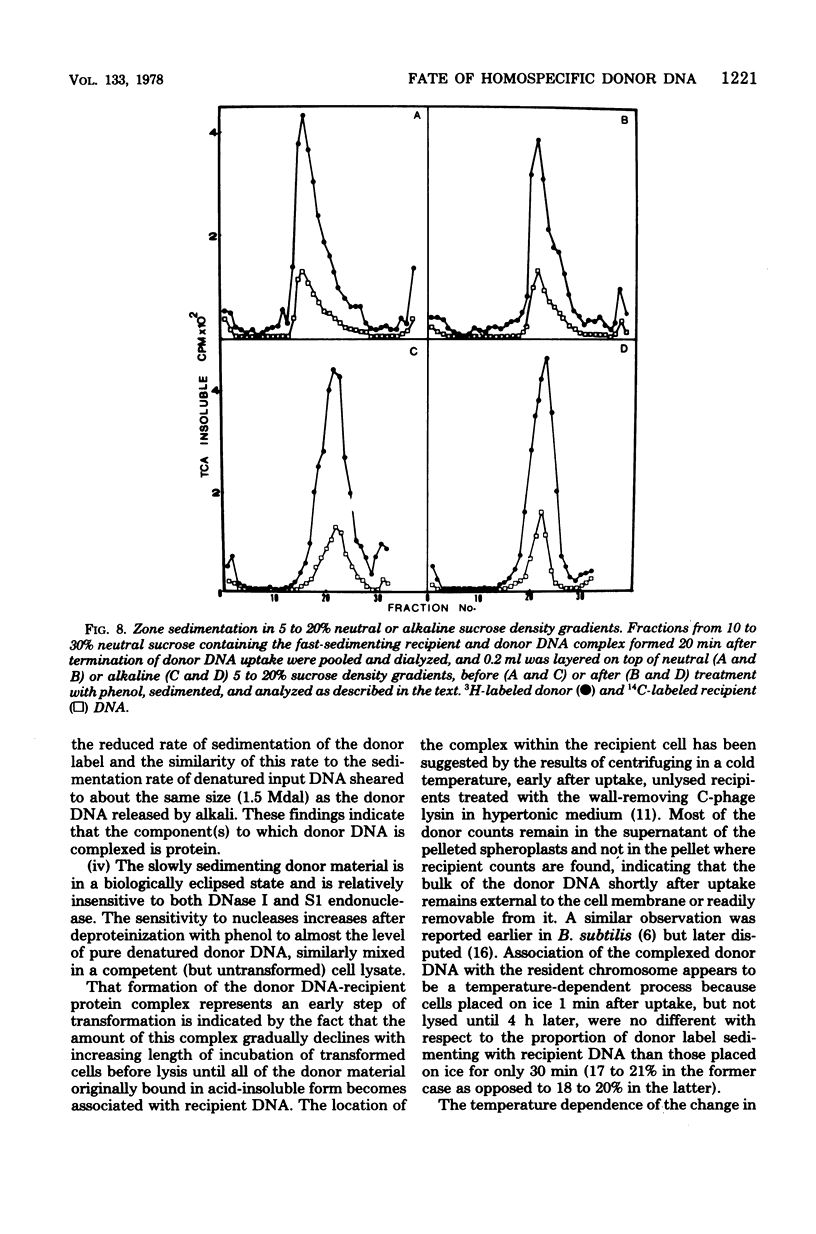
The fate of [3H]DNA from Streptococcus sanguis str-r43 fus-s donors in [14C]S. sanguis str-s fus-r1 recipients was studied by examining the lysates prepared from such recipients at various times after 1 min of exposure to DNA. The lysates were analyzed in CsCl and 10 to 30% sucrose gradients; fractions from the gradients were tested for biological activity and sensitivity to nucleases, subjected to various treatments and retested for nuclease sensitivity, and run on 5 to 20% neutral and alkaline sucrose gradients. The results demonstrate that donor DNA bound to S. sanguis cells in a form resistant to exogenous deoxyribonuclease is initially single stranded and complexed to recipient material. Donor DNA can be removed from the complex upon treatment of the complex with Pronase, phenol, or isoamyl alcohol-chloroform. Within the complex, donor DNA is relatively insensitive to S1 endonuclease but can regain its sensitivity by treatment with phenol. With time the complex moves as a whole to associate physically with the recipient chromosome. After a noncovalent stage of synapsis, donor material is covalently bonded to and acquires the nuclease sensitivity of recipient DNA, while donor markers regain transforming activity and become linked to resident markers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKULIS S. S., SMITH C., BOLTRALIK J. J., HEYMANN H. STRUCTURE OF STREPTOCOCCAL CELL WALLS. IV. PURIFICATION AND PROPERTIES OF STREPTOCOCCAL PHAGE MURALYSIN. J Biol Chem. 1964 Dec;239:4027–4033. [PubMed] [Google Scholar]
- Burgi A. W., Robinton J., Carlson C. L. Studies on the folded chromosome of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1974;38:43–51. doi: 10.1101/sqb.1974.038.01.007. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. Formation and properties of products isolated from transformed cells which are derived entirely from donor DNA. J Mol Biol. 1972 Feb 28;64(1):9–29. doi: 10.1016/0022-2836(72)90318-x. [DOI] [PubMed] [Google Scholar]
- FOX M. S., ALLEN M. K. ON THE MECHANISM OF DEOXYRIBONUCLEATE INTEGRATION IN PNEUMOCOCCAL TRANSFORMATION. Proc Natl Acad Sci U S A. 1964 Aug;52:412–419. doi: 10.1073/pnas.52.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B., Neuberger M. Role of a deoxyribonuclease in the genetic transformation of Diplococcus pneumoniae. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2305–2309. doi: 10.1073/pnas.71.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in pneumococcus: existence and properties of a complex involving donor deoxyribonucleate single strands in eclipse. J Bacteriol. 1977 Nov;132(2):576–583. doi: 10.1128/jb.132.2.576-583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettijohn D. E., Hecht R. RNA molecules bound to the folded bacterial genome stabilize DNA folds and segregate domains of supercoiling. Cold Spring Harb Symp Quant Biol. 1974;38:31–41. doi: 10.1101/sqb.1974.038.01.006. [DOI] [PubMed] [Google Scholar]
- Piechowska M., Fox M. S. Fate of transforming deoxyribonucleate in Bacillus subtilis. J Bacteriol. 1971 Nov;108(2):680–689. doi: 10.1128/jb.108.2.680-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVIN A. W., DESA J. H. GENETIC LINKAGE OF MUTATIONAL SITES AFFECTING SIMILAR CHARACTERS IN PNEUMOCOCCUS AND STREPTOCOCCUS. J Bacteriol. 1964 Jan;87:86–96. doi: 10.1128/jb.87.1.86-96.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina J. L., Metzer E., Ravin A. W. Fate of heterospecific transforming DNA bound to Streptococcus sanguis. J Bacteriol. 1978 Mar;133(3):1224–1231. doi: 10.1128/jb.133.3.1224-1231.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Guild W. R. Kinetics of integration of transforming DNA in pneumococcus. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3331–3335. doi: 10.1073/pnas.69.11.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Competence in Bacillus subtilis transformation system. Nature. 1967 Feb 25;213(5078):773–775. doi: 10.1038/213773a0. [DOI] [PubMed] [Google Scholar]



