Abstract
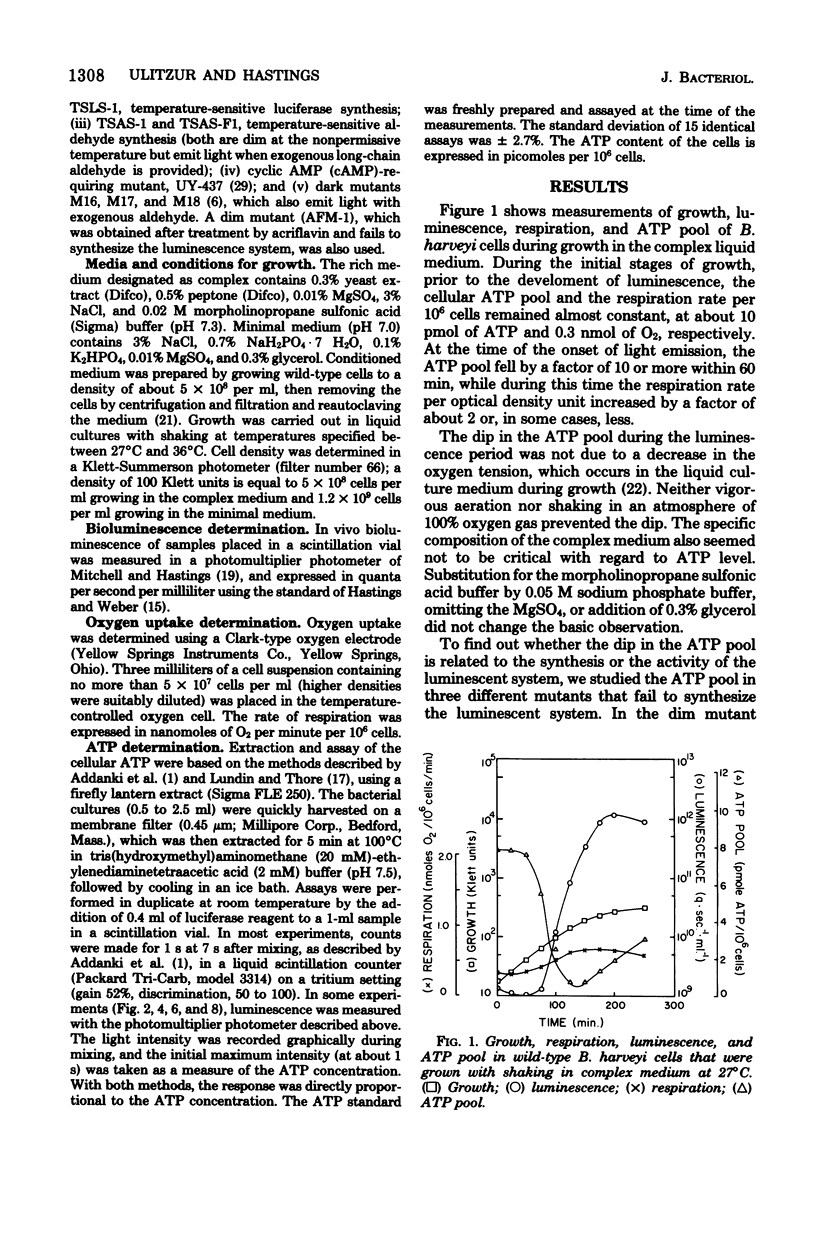
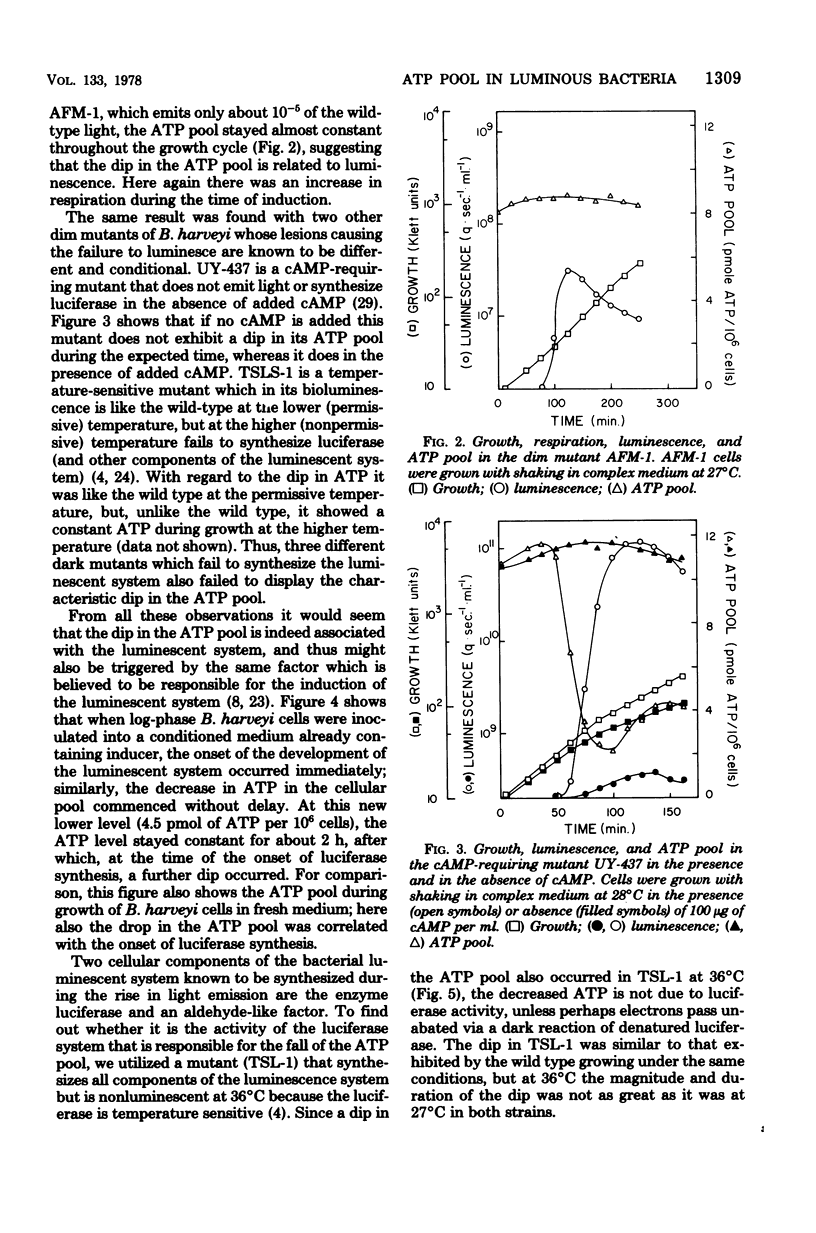
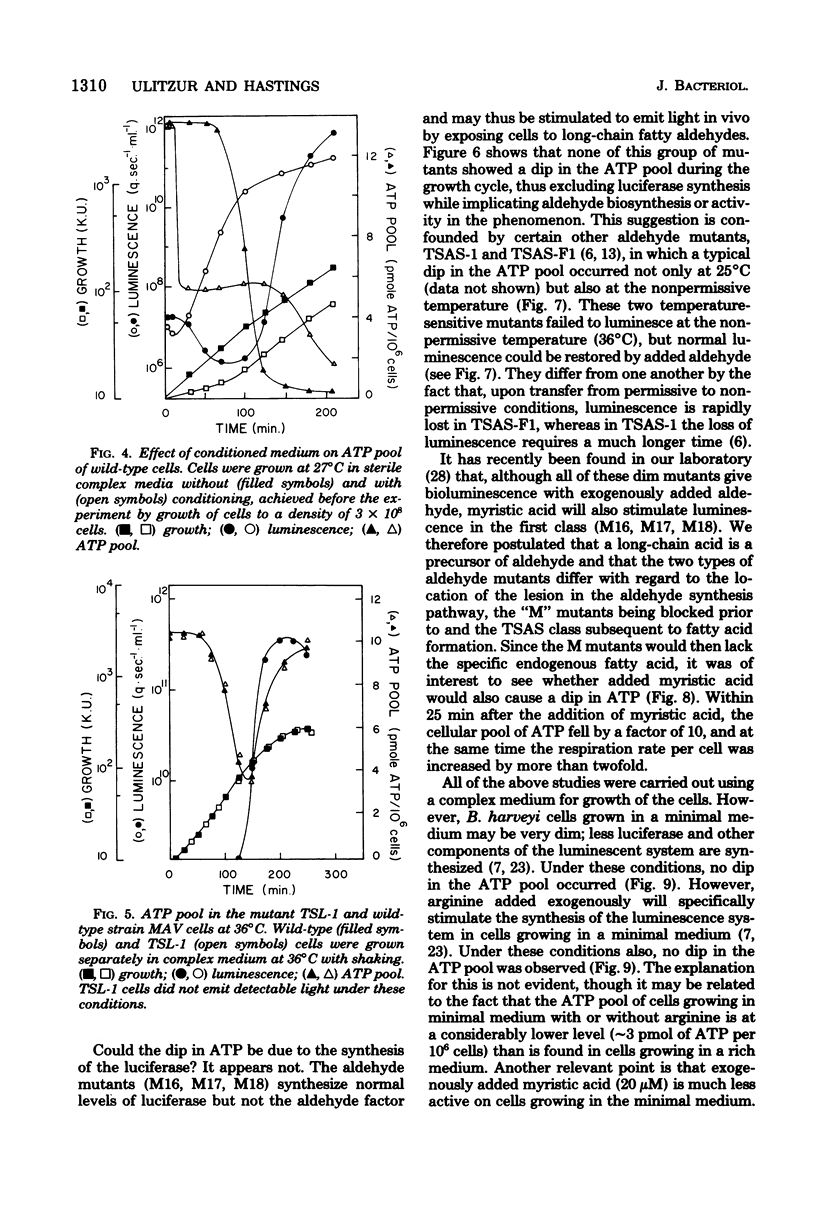
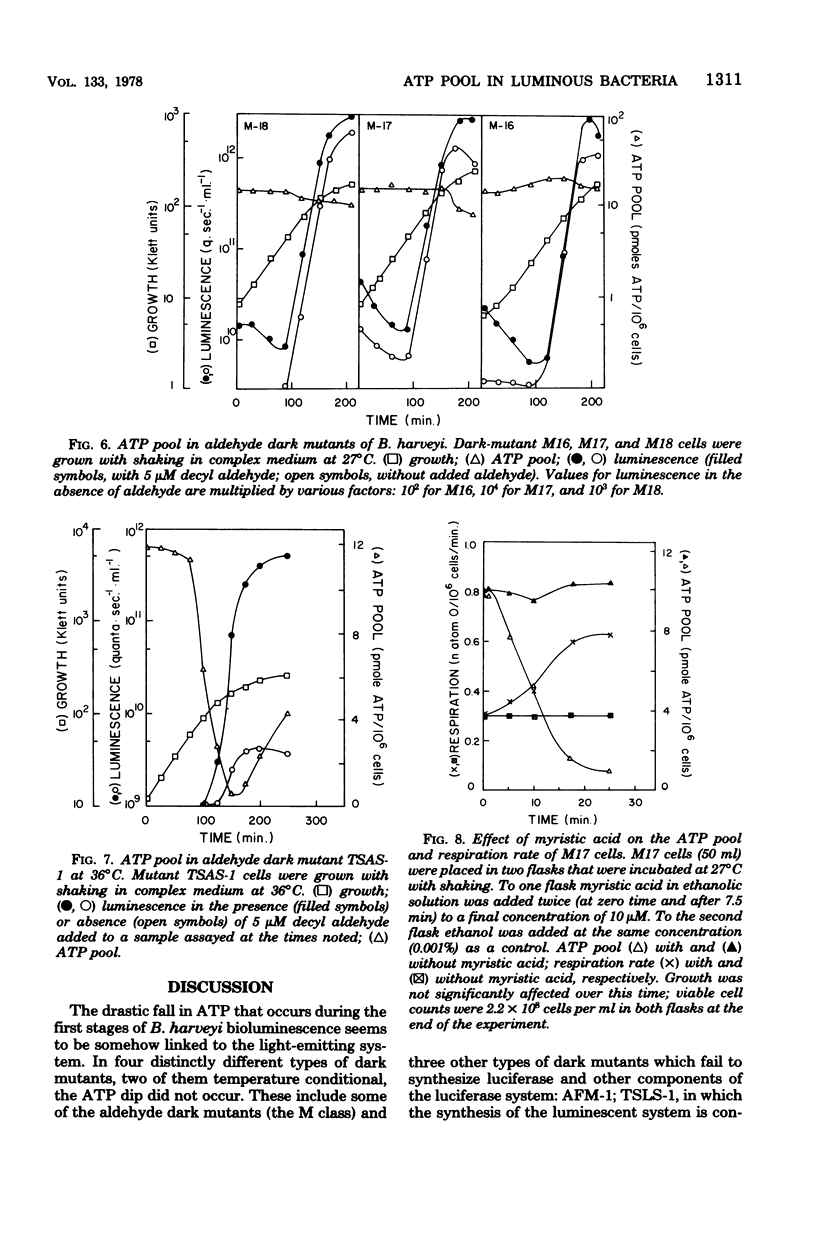
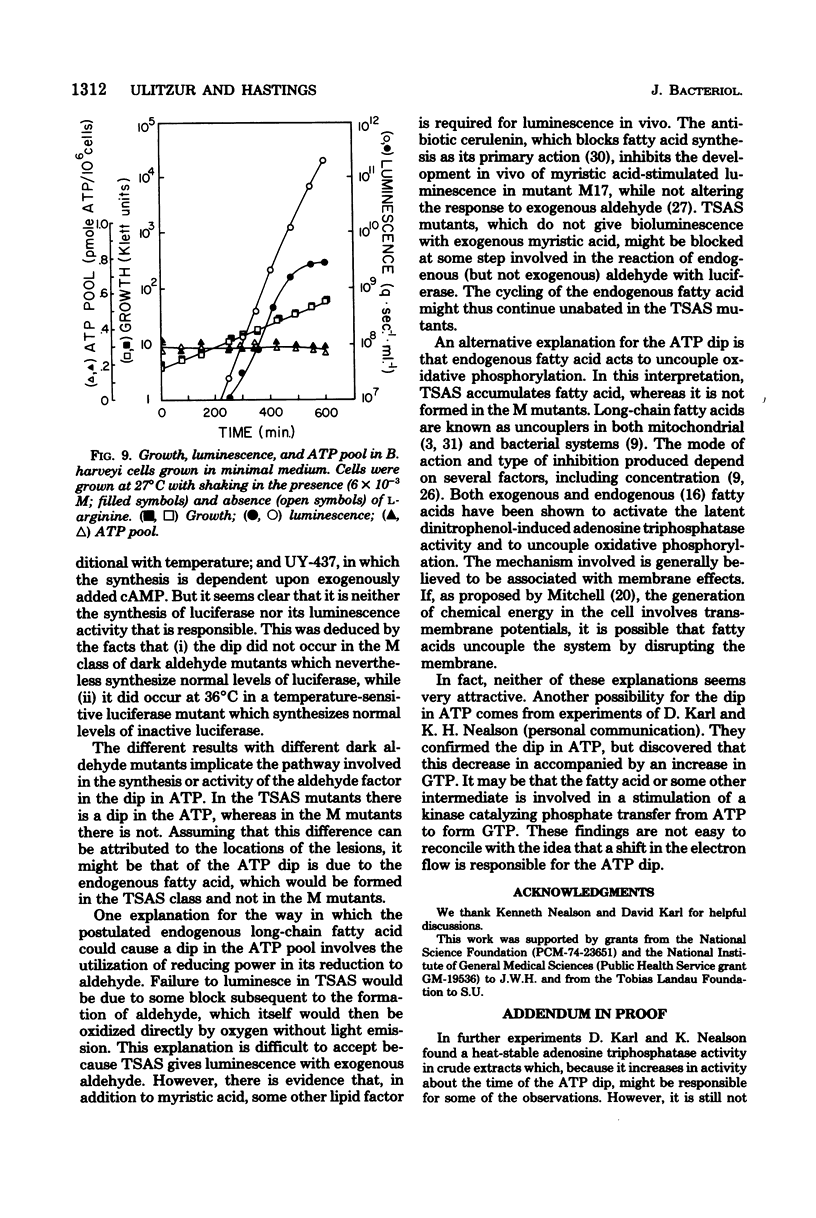
The bacterial bioluminescence system is unusual because it is self-induced. In the late logarithmic phase of growth, upon the accumulation of an autoinducer, the synthesis of the components of the system is initiated. We were interested in determining what effect this burst of synthesis and activity has on cellular energy metabolism. The ATP pool of the luminous bacterium Beneckea harveyi was found to dip 10- to 20-fold during the luminescence period, while the respiration per unit cell mass (optical density) increased but by much less. The dip in the ATP pool did not occur in four different types of dark mutants, including one that was temperature conditional and another that was conditional upon added cyclic AMP for luminescence. However, it is neither the synthesis nor the activity of luciferase that is responsible for the ATP dip; the dip does not occur in certain dark "aldehyde" mutants which nevertheless synthesize normal levels of luciferase, whereas it does occur at 36 degrees C in a temperature-sensitive luciferase mutant which forms normal levels of inactive luciferase. Results with other aldehyde mutants implicate the pathway involved in the synthesis of the aldehyde factor with the ATP dip.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addanki S., Sotos J. F., Rearick P. D. Rapid determination of picomole quantities of ATP with a liquid scintillation counter. Anal Biochem. 1966 Feb;14(2):261–264. doi: 10.1016/0003-2697(66)90135-7. [DOI] [PubMed] [Google Scholar]
- BORST P., LOOS J. A., CHRIST E. J., SLATER E. C. Uncoupling activity of long-chain fatty acids. Biochim Biophys Acta. 1962 Aug 27;62:509–518. doi: 10.1016/0006-3002(62)90232-9. [DOI] [PubMed] [Google Scholar]
- Cline T. W., Hastings J. W. Bacterial bioluminescence in vivo: control and synthesis of aldehyde factor in temperature-conditional luminescence mutants. J Bacteriol. 1974 Jun;118(3):1059–1066. doi: 10.1128/jb.118.3.1059-1066.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T. W., Hastings J. W. Mutationally altered bacterial luciferase. Implications for subunit functions. Biochemistry. 1972 Aug 29;11(18):3359–3370. doi: 10.1021/bi00768a008. [DOI] [PubMed] [Google Scholar]
- Cline T., Hastings J. W. Temperature-sensitive mutants of bioluminescent bacteria. Proc Natl Acad Sci U S A. 1971 Feb;68(2):500–504. doi: 10.1073/pnas.68.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey J. J. Inducible synthesis of bacterial luciferase: specificity and kinetics of induction. J Bacteriol. 1967 Nov;94(5):1638–1647. doi: 10.1128/jb.94.5.1638-1647.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard A. Inhibition and activation of bacterial luciferase synthesis. J Bacteriol. 1972 Mar;109(3):1101–1105. doi: 10.1128/jb.109.3.1101-1105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith H., Miller T. B. Effect of long chain fatty acids on bacterial respiration and amino acid uptake. J Appl Bacteriol. 1973 Dec;36(4):659–675. doi: 10.1111/j.1365-2672.1973.tb04151.x. [DOI] [PubMed] [Google Scholar]
- HASTINGS J. W., RILEY W. H., MASSA J. THE PURIFICATION PROPERTIES, AND CHEMILUMINESCENT QUANTUM YIELD OF BACTERIAL LUCIFERASE. J Biol Chem. 1965 Mar;240:1473–1481. [PubMed] [Google Scholar]
- HULSMANN W. C., ELLIOTT W. B., SLATER E. C. The nature and mechanism of action of uncoupling agents present in mitochrome preparations. Biochim Biophys Acta. 1960 Apr 8;39:267–276. doi: 10.1016/0006-3002(60)90163-3. [DOI] [PubMed] [Google Scholar]
- Hastings J. W. Bioluminescence: from chemical bonds to photons. Ciba Found Symp. 1975;(31):125–146. doi: 10.1002/9780470720134.ch8. [DOI] [PubMed] [Google Scholar]
- Hastings J. W., Nealson K. H. Bacterial bioluminescence. Annu Rev Microbiol. 1977;31:549–595. doi: 10.1146/annurev.mi.31.100177.003001. [DOI] [PubMed] [Google Scholar]
- Lundin A., Thore A. Analytical information obtainable by evaluation of the time course of firefly bioluminescence in the assay of ATP. Anal Biochem. 1975 May 26;66(1):47–63. doi: 10.1016/0003-2697(75)90723-x. [DOI] [PubMed] [Google Scholar]
- Michaliszyn G. A., Meighen E. A. Induced polypeptide synthesis during the development of bacterial bioluminescence. J Biol Chem. 1976 May 10;251(9):2541–2549. [PubMed] [Google Scholar]
- Mitchell G. W., Hastings J. W. A stable, inexpensive, solid-state photomultiplier photometer. Anal Biochem. 1971 Jan;39(1):243–250. doi: 10.1016/0003-2697(71)90481-7. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Ne'eman Z., Ulitzur S., Branton D., Hastings J. W. Membrane polypeptides co-induced with the bacterial bioluminescent system. J Biol Chem. 1977 Jul 25;252(14):5150–5154. [PubMed] [Google Scholar]
- Nealson K. H. Autoinduction of bacterial luciferase. Occurrence, mechanism and significance. Arch Microbiol. 1977 Feb 4;112(1):73–79. doi: 10.1007/BF00446657. [DOI] [PubMed] [Google Scholar]
- Nealson K. H., Hastings J. W. Low oxygen is optimal for luciferase synthesis in some bacteria. Ecological implications. Arch Microbiol. 1977 Feb 4;112(1):9–16. doi: 10.1007/BF00446648. [DOI] [PubMed] [Google Scholar]
- Nealson K. H., Platt T., Hastings J. W. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970 Oct;104(1):313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur S., Goldberg I. Sensitive, rapid, and specific bioassay for the determination of antilipogenic compounds. Antimicrob Agents Chemother. 1977 Sep;12(3):308–313. doi: 10.1128/aac.12.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur S., Hastings J. W. Myristic acid stimulation of bacterial bioluminescence in "aldehyde" mutants. Proc Natl Acad Sci U S A. 1978 Jan;75(1):266–269. doi: 10.1073/pnas.75.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur S., Yashphe J. An adenosine 3',5'-monophosphate-requiring mutant of the luminous bacteria Beneckea harveyi. Biochim Biophys Acta. 1975 Oct 9;404(2):321–328. doi: 10.1016/0304-4165(75)90339-6. [DOI] [PubMed] [Google Scholar]
- Vance D., Goldberg I., Mitsuhashi O., Bloch K. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem Biophys Res Commun. 1972 Aug 7;48(3):649–656. doi: 10.1016/0006-291x(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Mimura N., Takimoto A., Nakamura T. Luminescence and respiratory activities of Photobacterium phosphoreum. Competition for cellular reducing power. J Biochem. 1975 Jun;77(6):1147–1155. [PubMed] [Google Scholar]



