Abstract
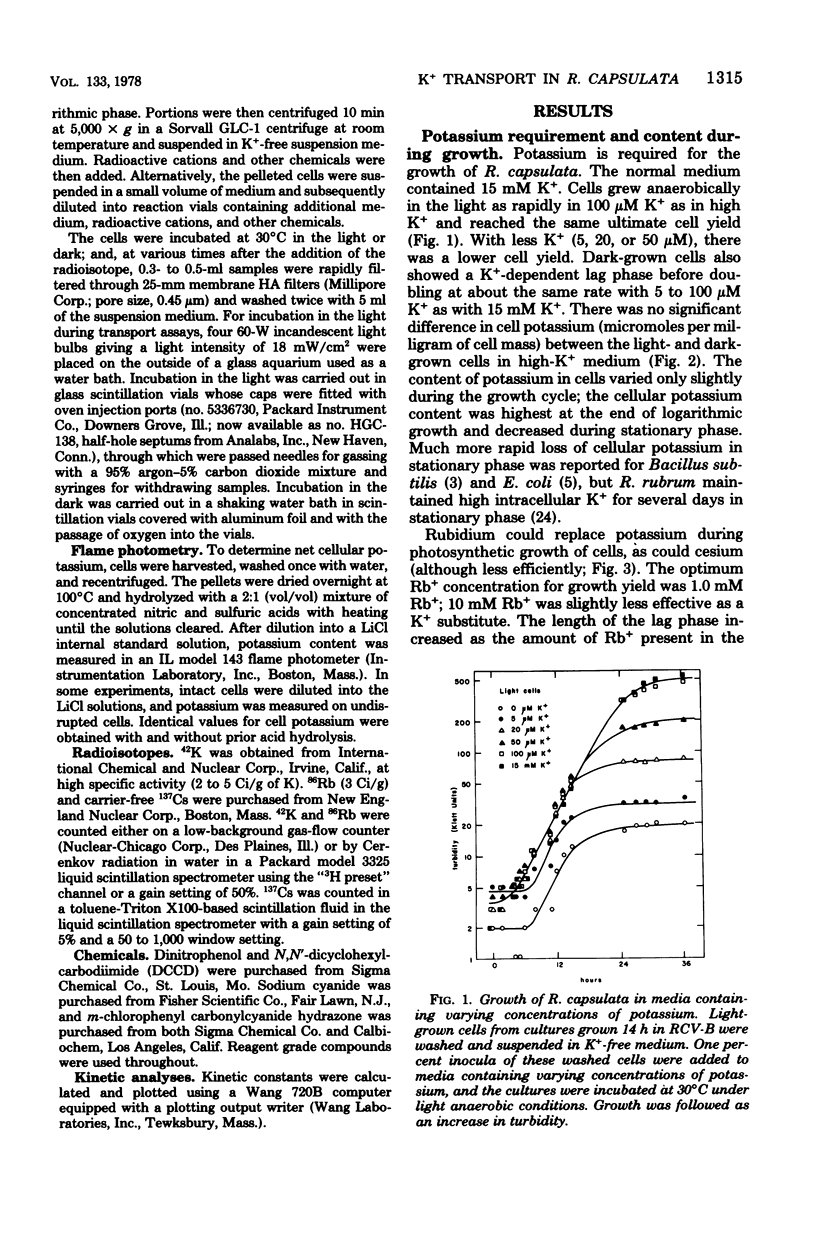
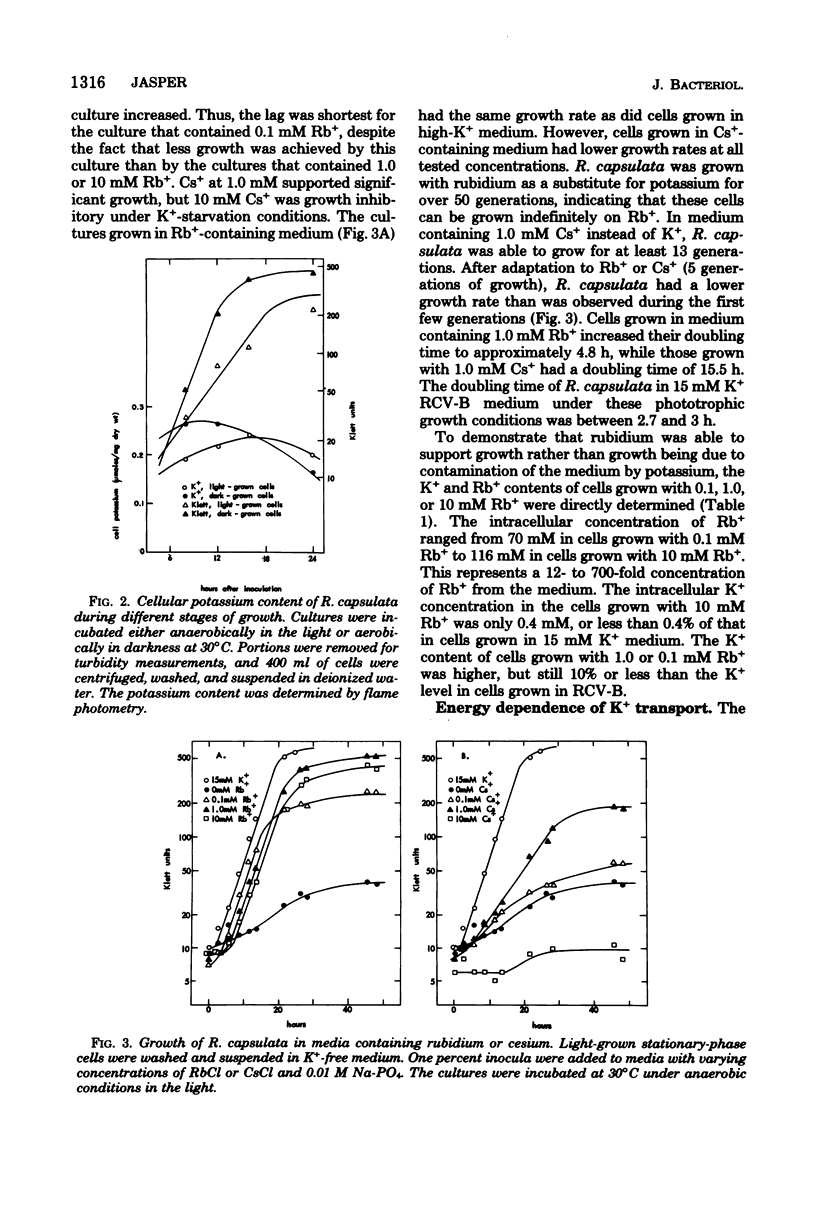
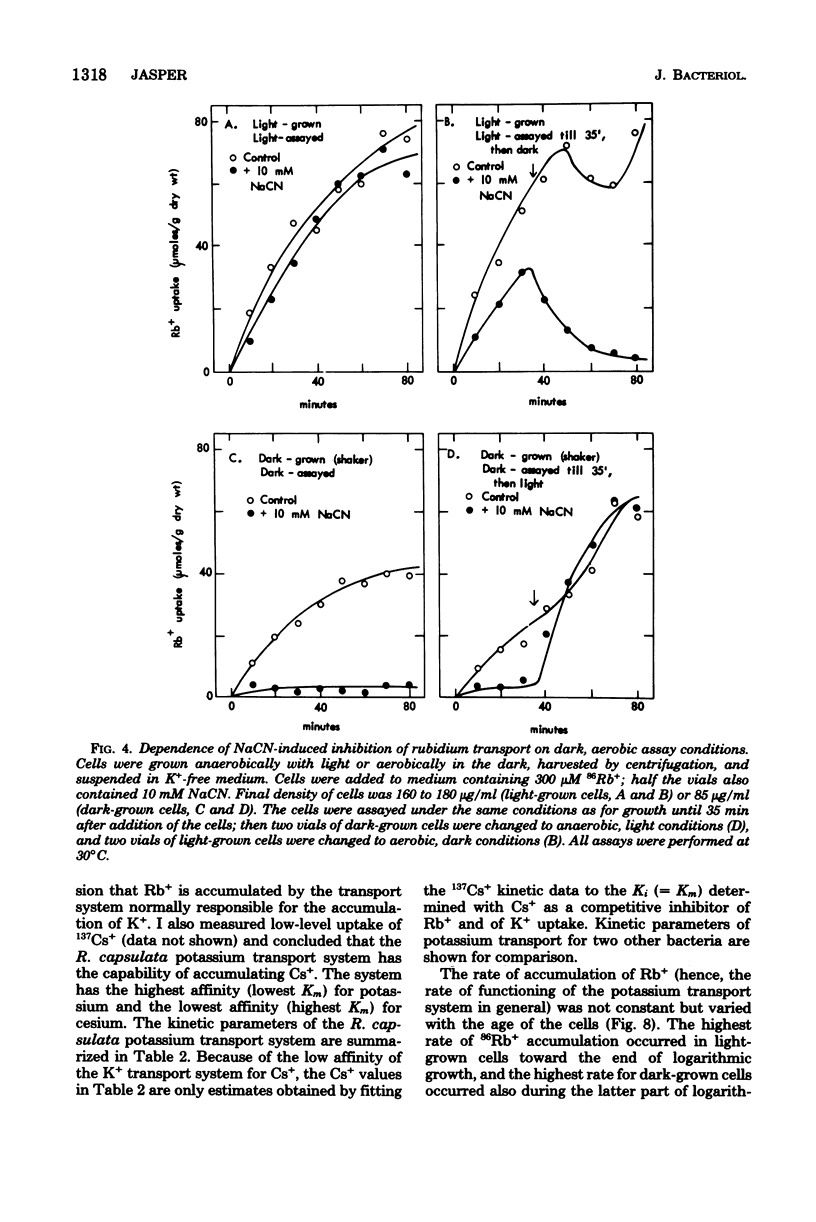
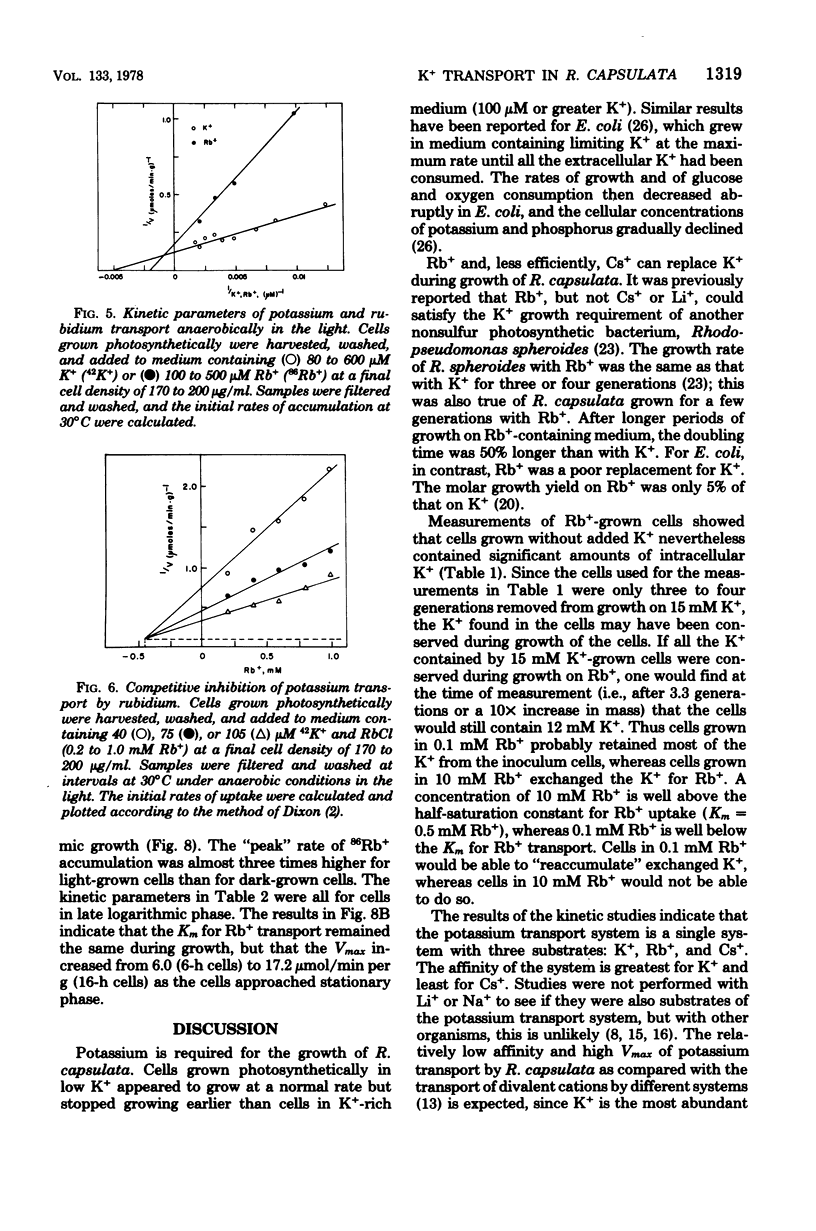
Rhodopseudomonas capsulata required potassium (or rubidium or cesium as analogs of potassium) for growth. These cations were actively accumulated by the cells by a process following Michaelis-Menten saturation kinetics. The monovalent cation transport system had Km's of 0.2 mM K+, 0.5 mM Rb+, and 2.6 mM Cs+. The rates of uptake of substrates by the potassium transport system varied with the age of the culture, although the affinity constant for the substrates remained constant. The maximal velocity of uptake of K+ was lower in aerobically grown cells than in photosynthetically grown cells, although the Km's for K+ and for Rb+ were about the same.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt E. Potassium content during growth and sporulation in Bacillus subtilis. J Bacteriol. 1972 Oct;112(1):264–267. doi: 10.1128/jb.112.1.264-267.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Kim B. S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Schultz S. G. Cation Transport in Escherichia coli: V. Regulation of cation content. J Gen Physiol. 1965 Nov 1;49(2):221–234. doi: 10.1085/jgp.49.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Schultz S. G. Cation transport in Escherichia coli. VI. K exchange. J Gen Physiol. 1966 Jan;49(3):469–481. doi: 10.1085/jgp.49.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELLER D. M. Oxidative phosphorylation in extracts of Rhodospirillum rubrum. J Biol Chem. 1962 Sep;237:2947–2954. [PubMed] [Google Scholar]
- HEYTLER P. G. uncoupling of oxidative phosphorylation by carbonyl cyanide phenylhydrazones. I. Some characteristics of m-Cl-CCP action on mitochondria and chloroplasts. Biochemistry. 1963 Mar-Apr;2:357–361. doi: 10.1021/bi00902a031. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of potassium transport by sodium in a mutant of Streptococcus faecalis. Biochemistry. 1967 Oct;6(10):3107–3110. doi: 10.1021/bi00862a018. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., MacLeod R. A. Kinetics of Na+-dependent K+ ion transport in a marine pseudomonad. J Bacteriol. 1975 Jan;121(1):160–164. doi: 10.1128/jb.121.1.160-164.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. B., Crofts A. R., von Stedingk L. V. Ion transport induced by light and antibiotics IN CHROMATOPHORES FROM Rhodospirillum rubrum. Eur J Biochem. 1968 Oct 17;6(1):41–54. doi: 10.1111/j.1432-1033.1968.tb00417.x. [DOI] [PubMed] [Google Scholar]
- La Monica R. F., Marrs B. L. The branched respiratory system of photosynthetically grown Rhodopseudomonas capsulata. Biochim Biophys Acta. 1976 Mar 12;423(3):431–439. doi: 10.1016/0005-2728(76)90198-5. [DOI] [PubMed] [Google Scholar]
- MACLEOD R. A., ONOFREY E. Nutrition and metabolism of marine bacteria. III. The relation of sodium and potassium to growth. J Cell Physiol. 1957 Dec;50(3):389–401. doi: 10.1002/jcp.1030500305. [DOI] [PubMed] [Google Scholar]
- MacLEOD R. A., SNELL E. E. The effect of related ions on the potassium requirement of lactic acid bacteria. J Biol Chem. 1948 Oct;176(1):39–52. [PubMed] [Google Scholar]
- Marrs B., Gest H. Regulation of bacteriochlorophyll synthesis by oxygen in respiratory mutants of Rhodopseudomonas capsulata. J Bacteriol. 1973 Jun;114(3):1052–1057. doi: 10.1128/jb.114.3.1052-1057.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Pressman B. C. Effects of ionophorous antibiotics on the light-induced internal and external hydrogen ion changes and phosphorylation in bacterial chromatophores. Biochemistry. 1969 Apr;8(4):1360–1370. doi: 10.1021/bi00832a009. [DOI] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Rhoads D. B., Epstein W. Energy coupling to net K+ transport in Escherichia coli K-12. J Biol Chem. 1977 Feb 25;252(4):1394–1401. [PubMed] [Google Scholar]
- Rhoads D. B., Waters F. B., Epstein W. Cation transport in Escherichia coli. VIII. Potassium transport mutants. J Gen Physiol. 1976 Mar;67(3):325–341. doi: 10.1085/jgp.67.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Jun;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- Stenn K. S. Cation transport in a photosynthetic bacterium. J Bacteriol. 1968 Sep;96(3):862–864. doi: 10.1128/jb.96.3.862-864.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Na+ and K+ gradients and alpha-aminoisobutyric acid transport in a marine pseudomonad. J Biol Chem. 1973 Oct 25;248(20):7106–7111. [PubMed] [Google Scholar]
- Weiden P. L., Epstein W., Schultz S. G. Cation transport in Escherichia coli. VII. Potassium requirement for phosphate uptake. J Gen Physiol. 1967 Jul;50(6):1641–1661. doi: 10.1085/jgp.50.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarlengo M. H., Schultz S. G. Cation transport and metabolism in Streptococcus fecalis. Biochim Biophys Acta. 1966 Oct 10;126(2):308–320. doi: 10.1016/0926-6585(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Zilinsky J. W., Sojka G. A., Gest H. Energy charge regulation in photosynthetic bacteria. Biochem Biophys Res Commun. 1971 Mar 5;42(5):955–961. doi: 10.1016/0006-291x(71)90523-7. [DOI] [PubMed] [Google Scholar]



