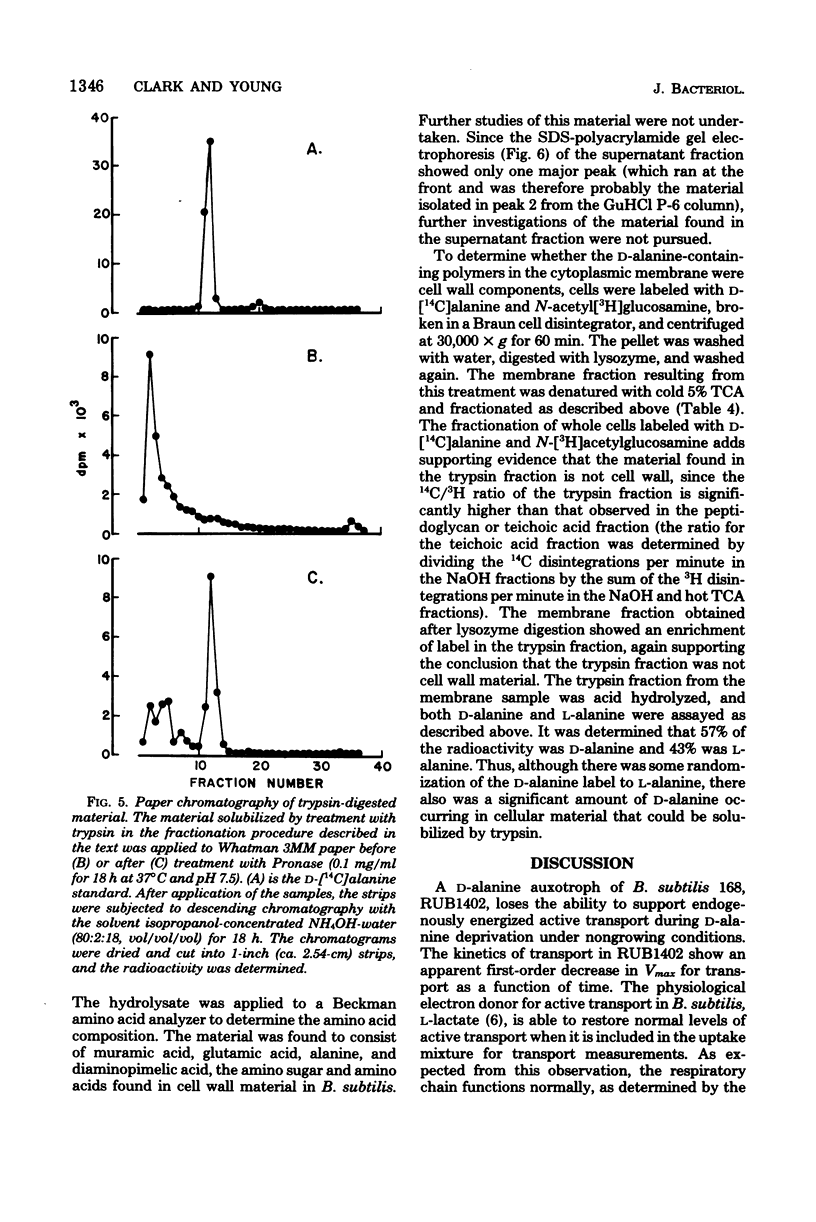
Abstract
An auxotroph of Bacillus subtilis 168 unable to synthesize D-alanine loses the ability to support endogenously energized transport when deprived of D-alanine. Revertants of the mutant retain transport activity. The loss of transport is specific for substrates taken up by active transport; substrates taken up by group translocation are transported at normal rates. The loss of transport can be retarded by pretreatment of the cells with inhibitors of protein synthesis. Since the loss of transport could be due to an alteration in a D-alanine-containing polymer, we investigated the incorporation of D-[14C]alanine into macromolecules. The major D-alanine-containing polymers in B. subtilis are peptidoglycan and teichoic acid, with 4 to 6% of the D-[14C]alanine label found in trypsin-soluble material. Whereas the peptidoglycan and teichoic acid undergo turnover, the trypsin-soluble material does not. Treatment of the trypsin-soluble material with Pronase releases free D-alanine. Analysis of acid-hydrolyzed trypsin-soluble material indicated that approximately 75% of the radioactivity is present as D-alanine, with the remainder present as L-alanine. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of partially purified D-[14C]alanine-labeled membranes indicated the presence of two peaks of radioactivity (molecular weights, 230,000 and 80,000) that could be digested by trypsin. The results suggest that D-alanine may be covalently bound to cellular proteins.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernlohr R. W., Clark V. Characterization and regulation of protease synthesis and activity in Bacillus licheniformis. J Bacteriol. 1971 Jan;105(1):276–283. doi: 10.1128/jb.105.1.276-283.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark V. L., Young F. E. Active transport of D-alanine and related amino acids by whole cells of Bacillus subtilis. J Bacteriol. 1974 Dec;120(3):1085–1092. doi: 10.1128/jb.120.3.1085-1092.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dul M. J., Young F. E. Genetic mapping of a mutant defective in D,L-alanine racemase in Bacillus subtilis 168. J Bacteriol. 1973 Sep;115(3):1212–1214. doi: 10.1128/jb.115.3.1212-1214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay P., Cordier P., Marquet M., Delobbe A. Carbohydrate metabolism and transport in Bacillus subtilis. A study of ctr mutations. Mol Gen Genet. 1973 Mar 19;121(4):355–368. doi: 10.1007/BF00433234. [DOI] [PubMed] [Google Scholar]
- Kaczorowski G., Shaw L., F-entes M., Walsh C. Coupling of alanine racemase and D-alanine dehydrogenase to active transport of amino acids in Escherichia coli B membrane vesicles. J Biol Chem. 1975 Apr 25;250(8):2855–2865. [PubMed] [Google Scholar]
- Konings W. N., Freese E. Amino acid transport in membrane vesicles of Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2408–2418. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linzer R., Neuhaus F. C. Biosynthesis of membrane teichoic acid. A role of the D-alanine-activating enzyme. J Biol Chem. 1973 May 10;248(9):3196–3201. [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Protease and peptidase activities in growing and sporulating cells and dormant spores of Bacillus megaterium. J Bacteriol. 1975 May;122(2):642–649. doi: 10.1128/jb.122.2.642-649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargel R. J., Shadur C. A., Neuhaus F. C. Mechanism of D-cycloserine action: transport systems for D-alanine, D-cycloserine, L-alanine, and glycine. J Bacteriol. 1970 Sep;103(3):778–788. doi: 10.1128/jb.103.3.778-788.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Autolytic enzyme associated with cell walls of Bacillus subtilis. J Biol Chem. 1966 Aug 10;241(15):3462–3467. [PubMed] [Google Scholar]


