Abstract
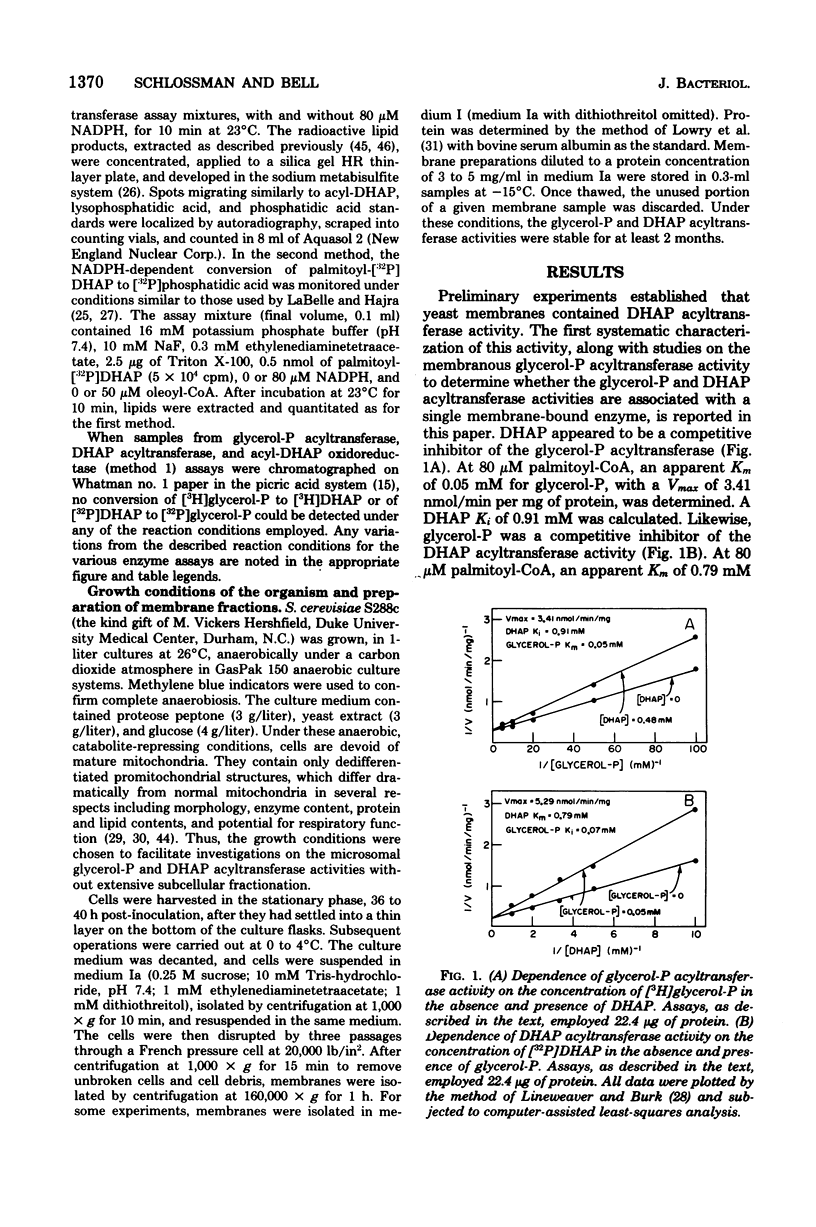
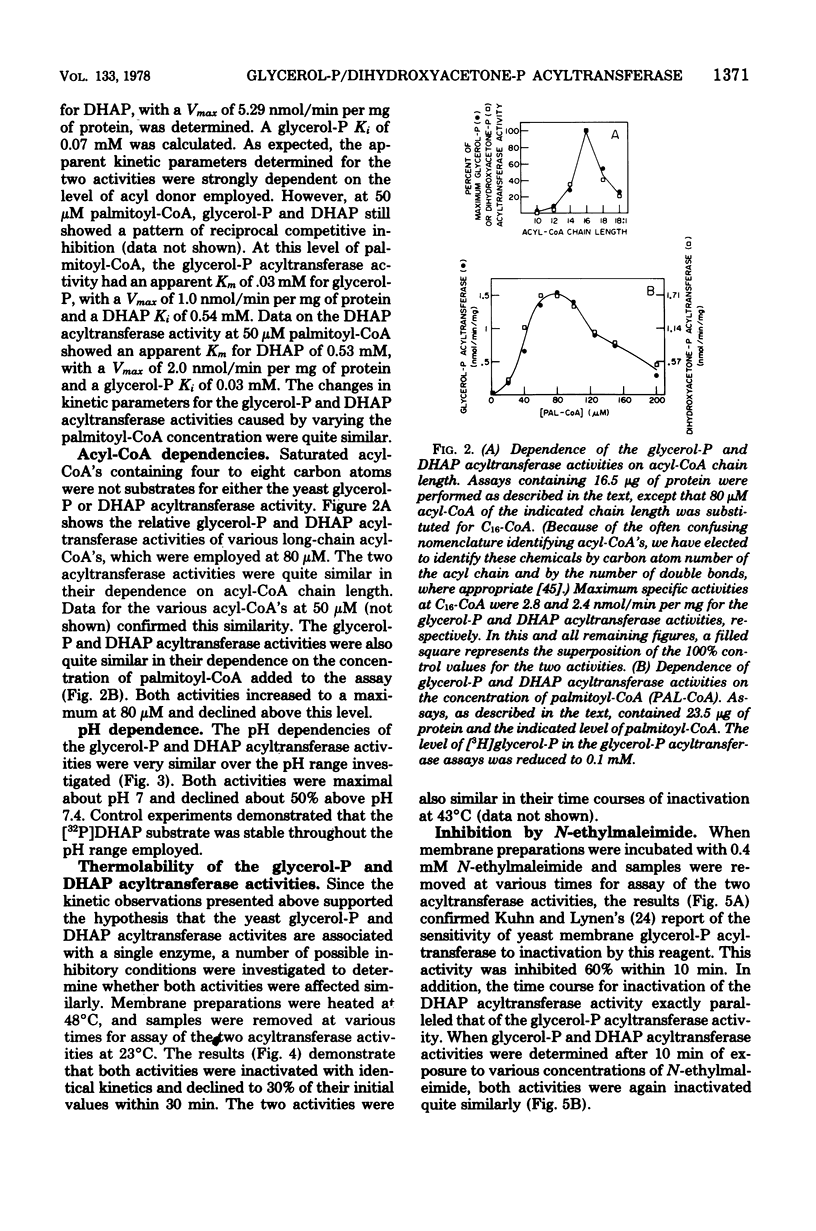
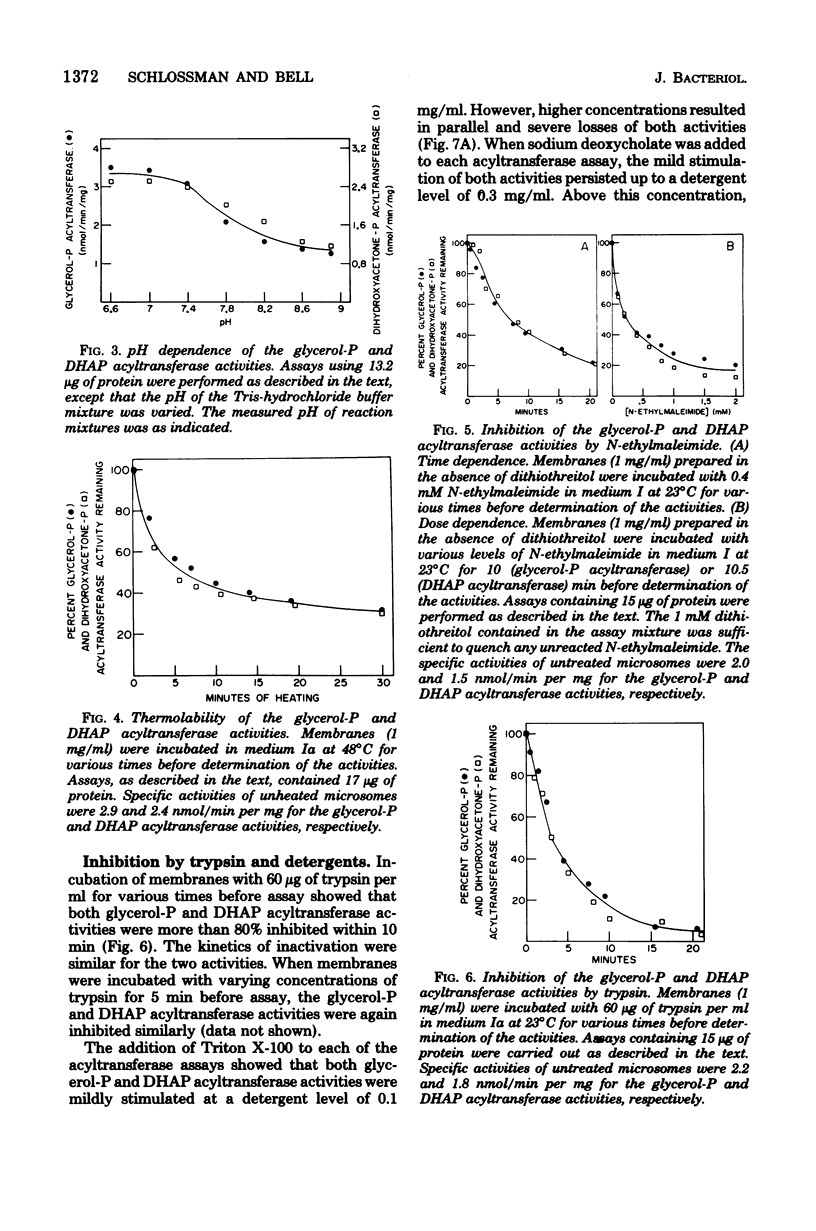
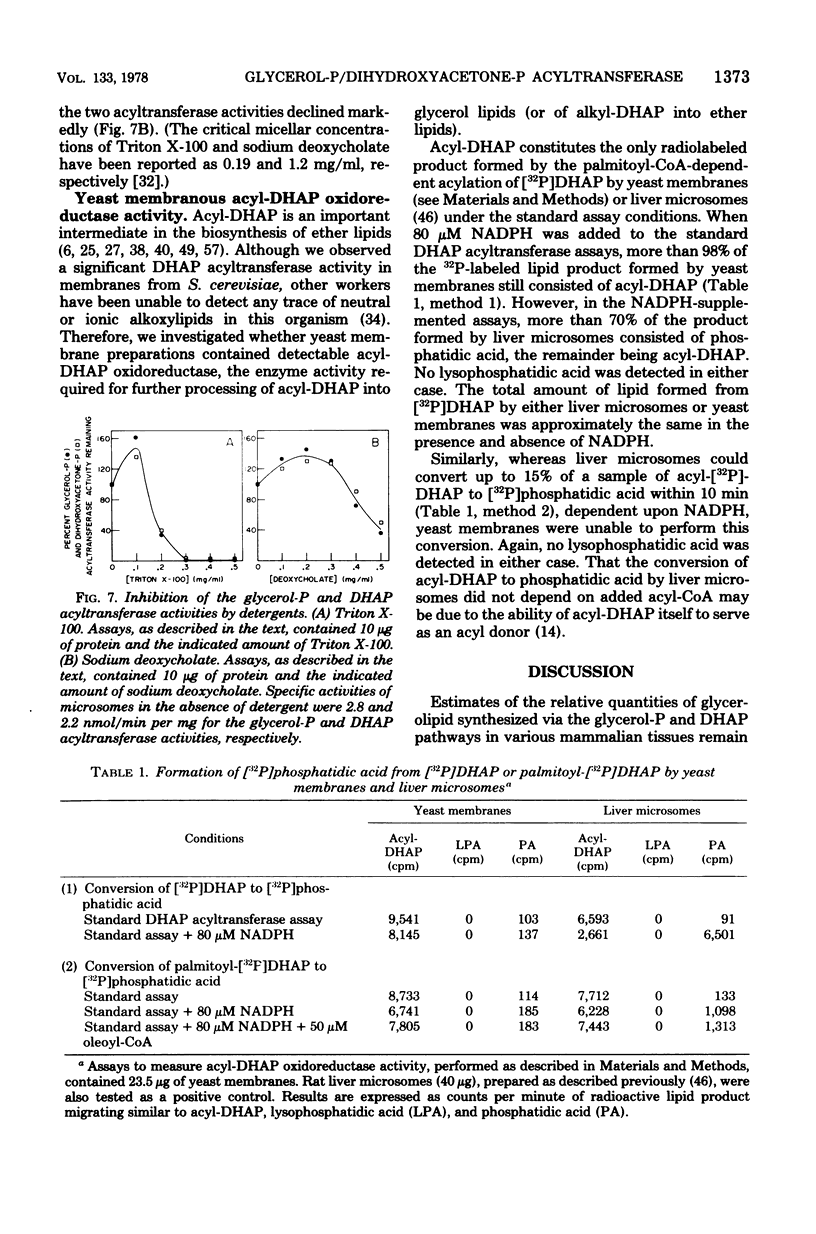
Yeast acyl-coenzyme A:dihydroxyacetone-phosphate O-acyltransferase (DHAP acyltransferase; EC 2.3.1.42) was investigated to (i) determine whether its activity and that of acyl-coenzyme A:sn-glycerol-3-phosphate O-acyltransferase (glycerol-P acyltransferase; EC 2.3.1.15) represent dual catalytic functions of a single membranous enzyme, (ii) estimate the relative contributions of the glycerol-P and DHAP pathways for yeast glycerolipid synthesis, and (iii) evaluate the suitability of yeast for future genetic investigations of the eucaryotic glycerol-P and DHAP acyltransferase activities. The membranous DHAP acyltransferase activity showed an apparent Km of 0.79 mM for DHAP, with a Vmax of 5.3 nmol/min per mg, whereas the glycerol-P acyltransferase activity showed an apparent Km of 0.05 mM for glycerol-P, with a Vmax of 3.4 nmol/min per mg. Glycerol-P was a competitive inhibitor (Ki, 0.07 mM) of the DHAP acyltransferase activity, and DHAP was a competitive inhibitor (Ki, 0.91 mM) of the glycerol-P acyltransferase activity. The two acyltransferase activities exhibited marked similarities in their pH dependence, acyl-coenzyme A chain length preference and substrate concentration dependencies, thermolability, and patterns of inactivation by N-ethylmaleimide, trypsin, and detergents. Thus, the data strongly suggest that yeast glycerol-P and DHAP acyltransferase activities represent dual catalytic functions of a single membrane-bound enzyme. Furthermore, since no acyl-DHAP oxidoreductase activity could be detected in yeast membranes, the DHAP pathway for glycerolipid synthesis may not operate in yeast.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W., Hajra A. K. The acyl dihydroxyacetone phosphate pathway for glycerolipid biosynthesis in mouse liver and Ehrlich ascites tumor cells. Proc Natl Acad Sci U S A. 1971 Feb;68(2):411–415. doi: 10.1073/pnas.68.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Ballard F. J. Effects of fasting and refeeding on the concentrations of glycolytic intermediates and the regulation of lipogenesis in rat adipose tissue in vivo. Biochim Biophys Acta. 1972 Jun 26;273(1):110–118. doi: 10.1016/0304-4165(72)90197-3. [DOI] [PubMed] [Google Scholar]
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis. Properties of wild type and Km defective sn-glycerol-3-phosphate acyltransferase activities. J Biol Chem. 1975 Sep 25;250(18):7147–7152. [PubMed] [Google Scholar]
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: macromolecular synthesis in an sn-glycerol 3-phosphate acyltransferase Km mutant. J Bacteriol. 1974 Mar;117(3):1065–1076. doi: 10.1128/jb.117.3.1065-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae K., Piantadosi C., Snyder F. Reductase, phosphatase, and kinase activities in the metabolism of alkyldihydroxyacetone phosphate and alkyldihydroxyacetone. J Biol Chem. 1973 Oct 10;248(19):6718–6723. [PubMed] [Google Scholar]
- Chang Y. Y., Kennedy E. P. Biosynthesis of phosphatidyl glycerophosphate in Escherichia coli. J Lipid Res. 1967 Sep;8(5):447–455. [PubMed] [Google Scholar]
- Cobon G. S., Crowfoot P. D., Linnane A. W. Biogenesis of mitchondria. Phospholipid synthesis in vitro by yeast mitochondrial and microsomal fractions. Biochem J. 1974 Nov;144(2):265–275. doi: 10.1042/bj1440265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curstedt T. Biosynthesis of molecular species of phosphatidylcholines in bile, liver and plasma of rats given (1,1-2H2)ethanol. Biochim Biophys Acta. 1974 Nov 18;369(2):196–208. doi: 10.1016/0005-2760(74)90251-3. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A. L., Gumaa K. A., McLean P. The distribution of hepatic metabolites and the control of the pathways of carbohydrate metabolism in animals of different dietary and hormonal status. Arch Biochem Biophys. 1971 Apr;143(2):617–663. doi: 10.1016/0003-9861(71)90247-5. [DOI] [PubMed] [Google Scholar]
- HOLZER H., BERNHARDT W., SCHNEIDER S. [On glycerin formation in baker's yeast]. Biochem Z. 1963;336:495–509. [PubMed] [Google Scholar]
- Hajra A. K., Agranoff B. W. Acyl dihydroxyacetone phosphate. Characterization of a 32P-labeled lipid from guinea pig liver mitochondria. J Biol Chem. 1968 Apr 10;243(7):1617–1622. [PubMed] [Google Scholar]
- Hajra A. K., Agranoff B. W. Reduction of palmitoyl dihydroxyacetone phosphate by mitochondria. J Biol Chem. 1968 Jun 25;243(12):3542–3543. [PubMed] [Google Scholar]
- Hajra A. K. Biosynthesis of acyl dihydroxyacetone phosphate in guinea pig liver mitochondria. J Biol Chem. 1968 Jun 25;243(12):3458–3465. [PubMed] [Google Scholar]
- Hajra A. K. Biosynthesis of phosphatidic acid from dihydroxyacetone phosphate. Biochem Biophys Res Commun. 1968 Dec 30;33(6):929–935. doi: 10.1016/0006-291x(68)90401-4. [DOI] [PubMed] [Google Scholar]
- Hajra A. K. Enzymatic transfer of the acyl group from acyl dihydroyxacetone phosphate to different substrates. Biochem Biophys Res Commun. 1974 Apr 8;57(3):668–674. doi: 10.1016/0006-291x(74)90598-1. [DOI] [PubMed] [Google Scholar]
- Hess B., Boiteux A., Krüger J. Cooperation of glycolytic enzymes. Adv Enzyme Regul. 1969;7:149–167. doi: 10.1016/0065-2571(69)90016-8. [DOI] [PubMed] [Google Scholar]
- Hill E. E., Lands W. E. Formation of acyl and alkenyl glycerol derivatives in Clostridium butyricum. Biochim Biophys Acta. 1970 Feb 10;202(1):209–211. doi: 10.1016/0005-2760(70)90239-0. [DOI] [PubMed] [Google Scholar]
- Johnston J. M., Paltauf F. Lipid metabolism in inositol-deficient yeast, Saccharomyces carlsbergensis. II. Incorporation of labeled precursors into lipids by whole cells and activities of some enzymes involved in lipid formation. Biochim Biophys Acta. 1970 Dec 15;218(3):431–440. [PubMed] [Google Scholar]
- KENNEDY E. P. Synthesis of phosphatides in isolated mitochondria. J Biol Chem. 1953 Mar;201(1):399–412. [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Enzymatic esterification of alpha-glycerophosphate by long chain fatty acids. J Biol Chem. 1953 Sep;204(1):345–357. [PubMed] [Google Scholar]
- KUHN N. J., LYNEN F. PHOSPHATIDIC ACID SYNTHESIS IN YEAST. Biochem J. 1965 Jan;94:240–246. doi: 10.1042/bj0940240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaBelle E. F., Jr, Hajra A. K. Biosynthesis of acyl dihydroxyacetone phosphate in subcellular fractions of rat liver. J Biol Chem. 1972 Sep 25;247(18):5835–5841. [PubMed] [Google Scholar]
- LaBelle E. F., Jr, Hajra A. K. Enzymatic reduction of alkyl and acyl derivatives of dihydroxyacetone phosphate by reduced pyridine nucleotides. J Biol Chem. 1972 Sep 25;247(18):5825–5834. [PubMed] [Google Scholar]
- LaBelle E. F., Jr, Hajra A. K. Purification and kinetic properties of acyl and alkyl dihydroxyacetone phosphate oxidoreductase. J Biol Chem. 1974 Nov 10;249(21):6936–6944. [PubMed] [Google Scholar]
- Linnane A. W., Lukins H. B. Isolation of mitochondria and techniques for studying mitochondrial biogenesis in yeasts. Methods Cell Biol. 1975;12:285–309. doi: 10.1016/s0091-679x(08)60961-9. [DOI] [PubMed] [Google Scholar]
- Makino S., Reynolds J. A., Tanford C. The binding of deoxycholate and Triton X-100 to proteins. J Biol Chem. 1973 Jul 25;248(14):4926–4932. [PubMed] [Google Scholar]
- Manning R., Brindley D. N. Tritium isotope effects in the measurement of the glycerol phosphate and dihydroxyacetone phosphate pathways of glycerolipid biosynthesis in rat liver. Biochem J. 1972 Dec;130(4):1003–1012. doi: 10.1042/bj1301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numa S., Yamashita S. Regulation of lipogenesis in animal tissues. Curr Top Cell Regul. 1974;8(0):197–246. doi: 10.1016/b978-0-12-152808-9.50012-2. [DOI] [PubMed] [Google Scholar]
- Pollock R. J., Hajra A. K., Agranoff B. W. Incorporation of D-[3-3H, U-14C] glucose into glycerolipid via acyl dihydroxyacetone phosphate untransformed and viral-transformed BHK-21-c13 fibroblasts. J Biol Chem. 1976 Sep 10;251(17):5149–5154. [PubMed] [Google Scholar]
- Pollock R. J., Hajra A. K., Agranoff B. W. The relative utilization of the acyl dihydroxyacetone phosphate and glycerol phosphate pathways for synthesis of glycerolipids in various tumors and normal tissues. Biochim Biophys Acta. 1975 Mar 24;380(3):421–435. doi: 10.1016/0005-2760(75)90110-1. [DOI] [PubMed] [Google Scholar]
- Pollock R. J., Hajra A. K., Folk W. R., Agranoff B. W. Use of (1 or 3-3H, U-14C)glucose to estimate the synthesis of glycerolipids via acyl dihydroxyacetone phosphate. Biochem Biophys Res Commun. 1975 Jul 22;65(2):658–664. doi: 10.1016/s0006-291x(75)80197-5. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Fitzgerald V., Snyder F. Properties of dihydroxyacetone phosphate acyltransferase in the harderian gland. J Biol Chem. 1977 Sep 25;252(18):6363–6366. [PubMed] [Google Scholar]
- Rognstad R., Clark D. G., Katz J. Pathways of glyceride glycerol synthesis. Biochem J. 1974 May;140(2):249–251. doi: 10.1042/bj1400249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANSLY P. G. Estimation of the enzymic condensation of alpha-glycerophosphate and palmityl coenzyme A. Biochim Biophys Acta. 1955 Nov;18(3):411–415. doi: 10.1016/0006-3002(55)90105-0. [DOI] [PubMed] [Google Scholar]
- Schlossman D. M., Bell R. M. Microsomal sn-glycerol 3-phosphate and dihydroxyacetone phosphate acyltransferase activities from liver and other tissues. Evidence for a single enzyme catalizing both reactions. Arch Biochem Biophys. 1977 Aug;182(2):732–742. doi: 10.1016/0003-9861(77)90555-0. [DOI] [PubMed] [Google Scholar]
- Schlossman D. M., Bell R. M. Triacylglycerol synthesis in isolated fat cells. Evidence that the sn-glycerol-3-phosphate and dihydroxyacetone phosphate acyltransferase activities are dual catalytic functions of a single microsomal enzyme. J Biol Chem. 1976 Sep 25;251(18):5738–5744. [PubMed] [Google Scholar]
- Silbert D. F. Genetic modification of membrane lipid. Annu Rev Biochem. 1975;44:315–339. doi: 10.1146/annurev.bi.44.070175.001531. [DOI] [PubMed] [Google Scholar]
- Snider M. D., Kennedy E. P. Partial purification of glycerophosphate acyltransferase from Escherichia coli. J Bacteriol. 1977 Jun;130(3):1072–1083. doi: 10.1128/jb.130.3.1072-1083.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M. R., Lester R. L. In vitro studies of phospholipid biosynthesis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Feb 21;260(2):222–243. doi: 10.1016/0005-2760(72)90035-5. [DOI] [PubMed] [Google Scholar]
- Tamai Y., Lands W. E. Positional specificity of sn-glycerol 3-phosphate acylation during phosphatidate formation by rat liver microsomes. J Biochem. 1974 Oct;76(4):847–860. [PubMed] [Google Scholar]
- White G. L., Hawthorne J. N. Phosphatidic acid and phosphatidylinositol metabolism in Schizosaccharomyces pombe. Biochem J. 1970 Apr;117(2):203–213. doi: 10.1042/bj1170203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykle R. L., Plantadosi C., Snyder F. The role of acyldihydroxyacetone phosphate, reduced nicotinamide adenine dinucleotide, and reduced nicotinamide adenine dinucleotide phosphate in the biosynthesis of O-alkyl glycerolipids by microsomal enzymes of Ehrlich ascites tumor. J Biol Chem. 1972 May 10;247(9):2944–2948. [PubMed] [Google Scholar]
- Yamashita S., Hosaka K., Numa S. Resolution and reconstitution of the phosphatidate-synthesizing system of rat-liver microsomes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3490–3492. doi: 10.1073/pnas.69.11.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S., Numa S. Partial purification and properties of glycerophosphate acyltransferase from rat liver. Formation of 1-acylglycerol 3-phosphate from sn-glycerol 3-phosphate and palmityl coenzyme A. Eur J Biochem. 1972 Dec 18;31(3):565–573. doi: 10.1111/j.1432-1033.1972.tb02566.x. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Phosphoglyceride metabolism. Annu Rev Biochem. 1974;43(0):243–277. doi: 10.1146/annurev.bi.43.070174.001331. [DOI] [PubMed] [Google Scholar]



