Abstract
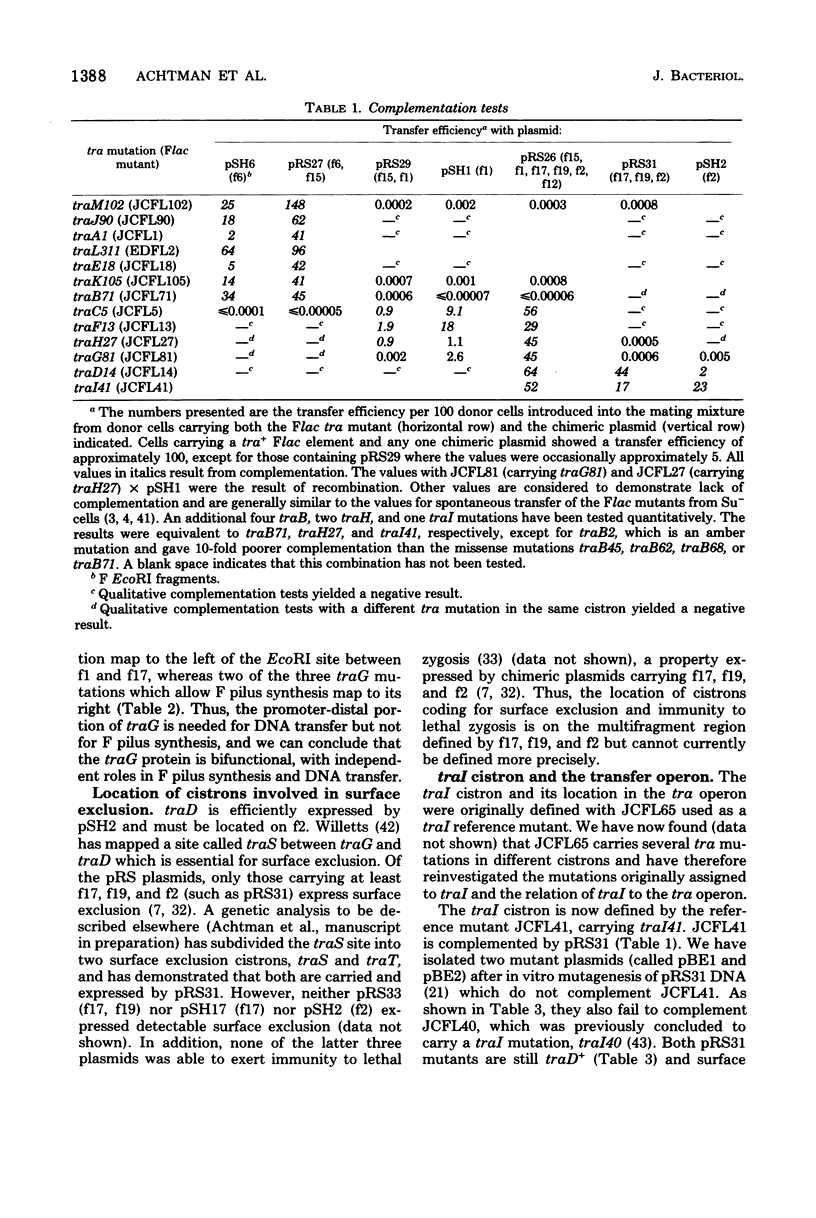
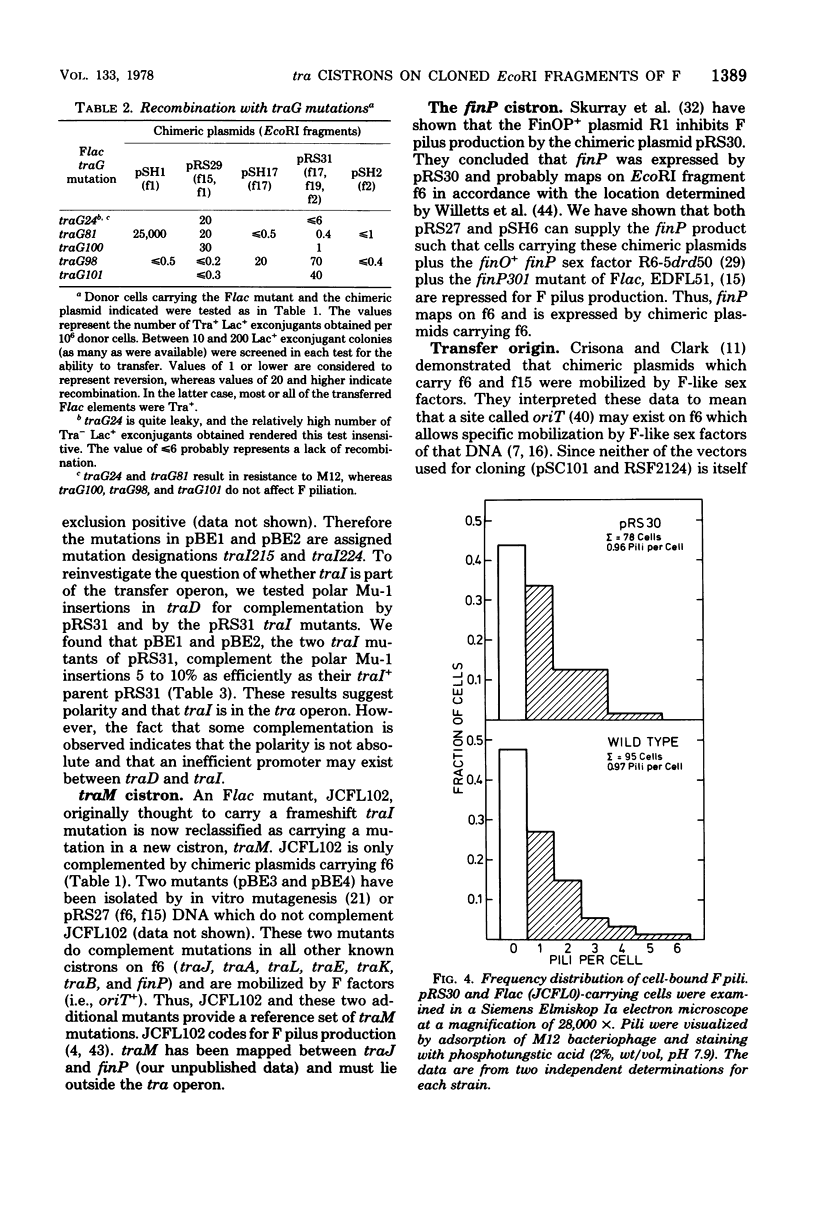
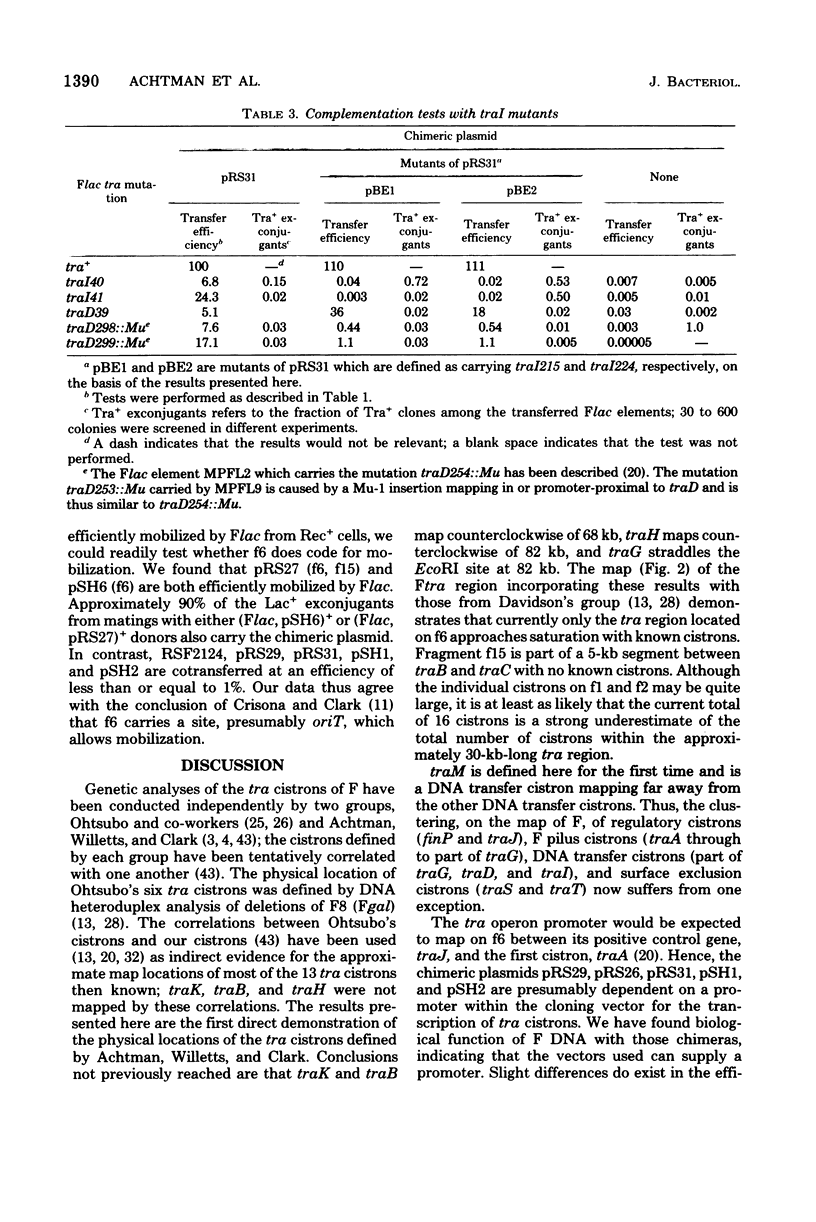
We describe here the cloning of single EcoRI fragments from the tra region of F DNA using ColE1::Tn3 as vector. These plasmids, as well as the series of Skurray et al. (Proc. Natl. Acad. Sci. U.S.A. 73:64-68, 1976), have been used to refine the map positions of tra cistrons on the F factor as well as to define a new DNA transfer cistron, traM. The current map of the tra cistrons is presented. None of the known tra cistrons, with the exception of traG, straddles an EcoRI site. The EcoRI site at 82 kilobases splits the traG cistron into two portions, an operator-proximal portion necessary for F pilus synthesis and an operator distal portion involved in conjugation itself. The operon structure of the tra cistrons was reevaluated, and we found that traI is at least partially independent of transcription of the traA to traD operon.
Full text
PDF

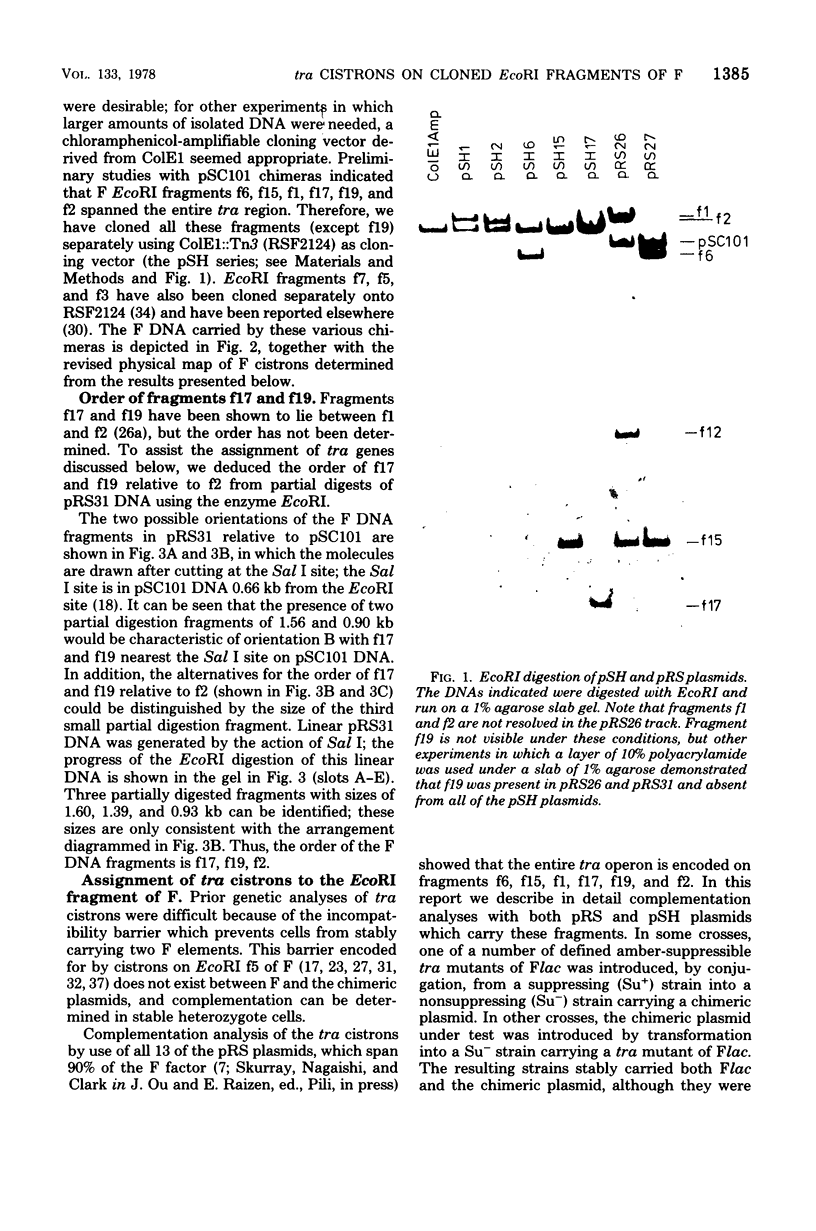
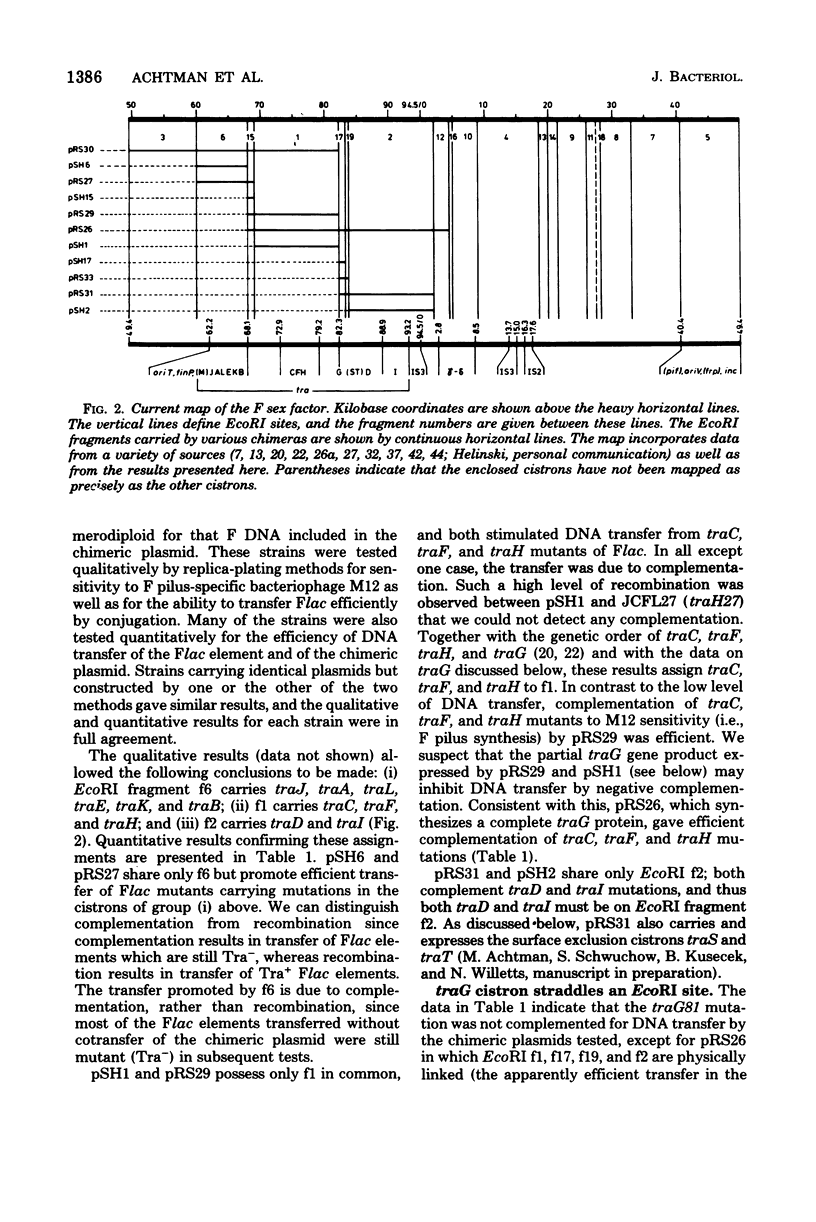
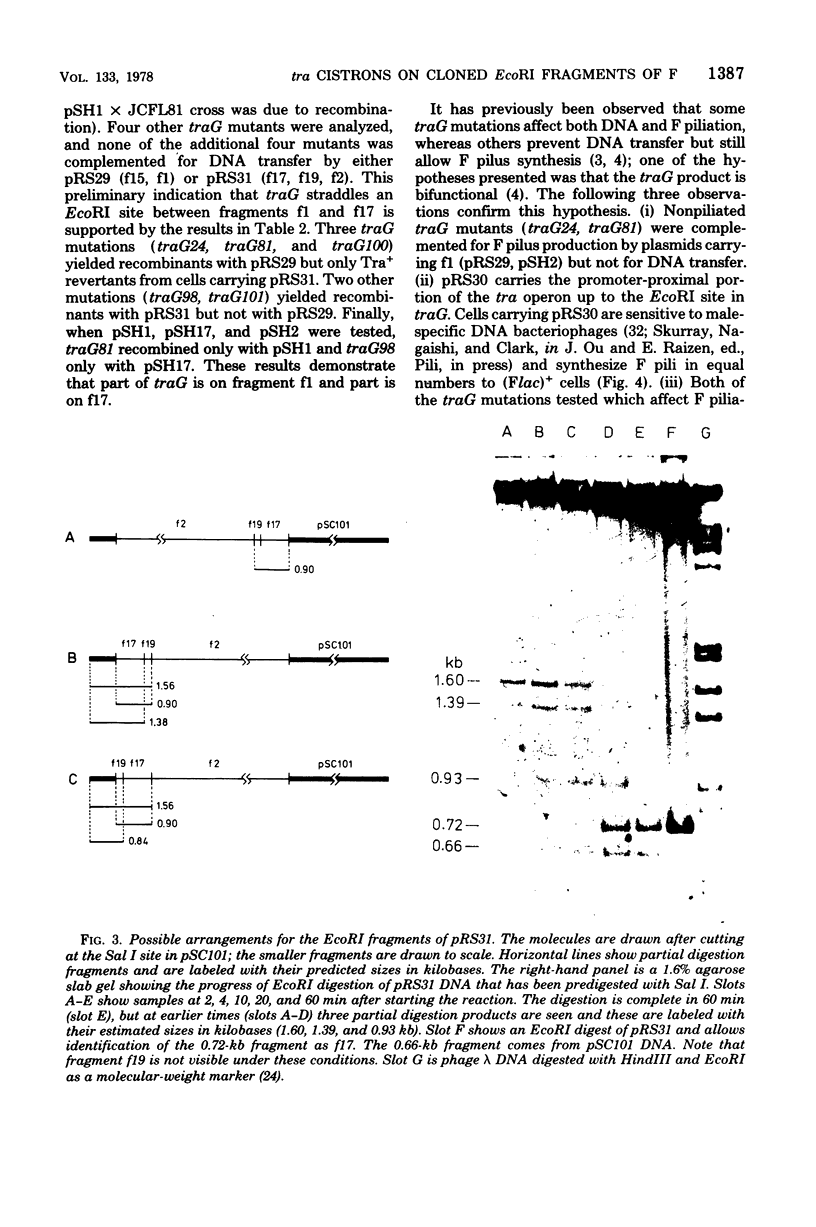


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M. Genetics of the F sex factor in enterobacteriaceae. Curr Top Microbiol Immunol. 1973;60:79–123. doi: 10.1007/978-3-642-65502-9_3. [DOI] [PubMed] [Google Scholar]
- Achtman M., Kennedy N., Skurray R. Cell--cell interactions in conjugating Escherichia coli: role of traT protein in surface exclusion. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5104–5108. doi: 10.1073/pnas.74.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Conjugational complementation analysis of transfer-deficient mutants of Flac in Escherichia coli. J Bacteriol. 1972 Jun;110(3):831–842. doi: 10.1128/jb.110.3.831-842.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony W. M., Deonier R. C., Lee H. J., Hu S., Otsubo E., Davidson N., Broda P. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. IX. Note on the deletion mutant of F, F delta(33-43). J Mol Biol. 1974 Nov 15;89(4):647–650. doi: 10.1016/0022-2836(74)90041-2. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Wilde L. C. Restriction of a bacteriophage of Streptomyces albus G involving endonuclease SalI. J Bacteriol. 1976 Nov;128(2):644–650. doi: 10.1128/jb.128.2.644-650.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisona N. J., Clark A. J. Increase in conjugational transmission frequency of nonconjugative plasmids. Science. 1977 Apr 8;196(4286):186–187. doi: 10.1126/science.322280. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J., Willetts N. S. Two classes of Flac mutants insensitive to transfer inhibition by an F-like R factor. Mol Gen Genet. 1971;111(3):256–264. doi: 10.1007/BF00433110. [DOI] [PubMed] [Google Scholar]
- Guyer M. S., Clark A. J. cis-Dominant, transfer-deficient mutants of the Escherichia coli K-12 F sex factor. J Bacteriol. 1976 Jan;125(1):233–247. doi: 10.1128/jb.125.1.233-247.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S., Figurski D., Davidson N. Electron microscope study of a plasmid chimera containing the replication region of the Escherichia coli F plasmid. J Bacteriol. 1976 Aug;127(2):988–997. doi: 10.1128/jb.127.2.988-997.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H., Thomas C. A., Jr Molecular cloning of DNA fragments produced by restriction endonucleases Sa1I and BamI. Proc Natl Acad Sci U S A. 1976 May;73(5):1537–1541. doi: 10.1073/pnas.73.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. S., Smith M. G. The chromosome of bacteriophage T5. I. Analysis of the single-stranded DNA fragments by agarose gel electrophoresis. J Mol Biol. 1972 Feb 14;63(3):383–395. doi: 10.1016/0022-2836(72)90435-4. [DOI] [PubMed] [Google Scholar]
- Helmuth R., Achtman M. Operon structure of DNA transfer cistrons on the F sex factor. Nature. 1975 Oct 23;257(5528):652–656. doi: 10.1038/257652a0. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Smith H. R., Anderson E. S. Mutagenesis of plasmid DNA with hydroxylamine: isolation of mutants of multi-copy plasmids. Mol Gen Genet. 1976 Apr 23;145(1):101–108. doi: 10.1007/BF00331564. [DOI] [PubMed] [Google Scholar]
- Ippen-Ihler K., Achtman M., Willetts N. Deletion map of the Escherichia coli K-12 sex factor F: the order of eleven transfer cistrons. J Bacteriol. 1972 Jun;110(3):857–863. doi: 10.1128/jb.110.3.857-863.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Helinski D. R. Method for the isolation of the replication region of a bacterial replicon: construction of a mini-F'kn plasmid. J Bacteriol. 1976 Aug;127(2):982–987. doi: 10.1128/jb.127.2.982-987.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Otsubo E. Transfer-defective mutants of sex factors in Escherichia coli. II. Deletion mutants of an F-prime and deletion mapping of cistrons involved in genetic transfer. Genetics. 1970 Feb;64(2):189–197. doi: 10.1093/genetics/64.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchaudhuri S., Maas W. K. Physical mapping of a DNA sequence common to plasmids of incompatibility group F I. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1190–1194. doi: 10.1073/pnas.74.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Cohen S. N. Nonchromosomal antibiotic resistance in bacteria. V. Isolation and characterization of R factor mutants exhibiting temperature-sensitive repression of fertility. J Bacteriol. 1972 Jun;110(3):1082–1088. doi: 10.1128/jb.110.3.1082-1088.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Guyer M. S., Timmis K., Cabello F., Cohen S. N., Davidson N., Clark A. J. Replication region fragments cloned from Flac+ are identical to EcoRI fragment f5 of F. J Bacteriol. 1976 Sep;127(3):1571–1575. doi: 10.1128/jb.127.3.1571-1575.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Nagaishi H., Clark A. J. Molecular cloning of DNA from F sex factor of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1976 Jan;73(1):64–68. doi: 10.1073/pnas.73.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Willetts N., Reeves P. Effect of tra mutations on F factor-specified immunity to lethal zygosis. Mol Gen Genet. 1976 Jul 23;146(2):161–165. doi: 10.1007/BF00268084. [DOI] [PubMed] [Google Scholar]
- So M., Gill R., Falkow S. The generation of a ColE1-Apr cloning vehicle which allows detection of inserted DNA. Mol Gen Genet. 1975 Dec 30;142(3):239–249. doi: 10.1007/BF00425649. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R., Hughes S. G., Broda P. Plasmid identification using specific endonucleases. Mol Gen Genet. 1974;133(2):141–149. doi: 10.1007/BF00264835. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G., Sherratt D. Complementation of transfer deficient ColE1 mutants. Mol Gen Genet. 1977 Mar 7;151(2):197–201. doi: 10.1007/BF00338695. [DOI] [PubMed] [Google Scholar]
- Willetts N. S. Characterization of the F transfer cistron, traL. Genet Res. 1973 Apr;21(2):205–213. doi: 10.1017/s0016672300013379. [DOI] [PubMed] [Google Scholar]
- Willetts N. S. Location of the origin of transfer of the sex factor F. J Bacteriol. 1972 Nov;112(2):773–778. doi: 10.1128/jb.112.2.773-778.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Achtman M. Genetic analysis of transfer by the Escherichia coli sex factor F, using P1 transductional complementation. J Bacteriol. 1972 Jun;110(3):843–851. doi: 10.1128/jb.110.3.843-851.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. Mapping loci for surface exclusion and incompatibility on the F factor of Escherichia coli K-12. J Bacteriol. 1974 Jun;118(3):778–782. doi: 10.1128/jb.118.3.778-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Maule J., McIntire S. The genetic locations of traO, finP and tra-4 on the E. coli K12 sex factor F. Genet Res. 1975 Dec;26(3):255–263. doi: 10.1017/s0016672300016050. [DOI] [PubMed] [Google Scholar]
- Willetts N. The genetics of transmissible plasmids. Annu Rev Genet. 1972;6:257–268. doi: 10.1146/annurev.ge.06.120172.001353. [DOI] [PubMed] [Google Scholar]