Summary
Wnt signaling plays important roles in cell polarization in diverse organisms, and loss of cell polarity is an early event in tumorigenesis caused by mutations in Wnt pathway genes. Despite this, the precise roles of Wnt proteins in cell polarization have remained elusive. In no organism has it been shown that the asymmetric position of a Wnt signal is essential to establishing a cell’s polarity. Attempts to test this by ubiquitous expression of Wnt genes have suggested that Wnt signals might act only as permissive factors in cell polarization. Here we find, using cell manipulations and ectopic gene expression in C. elegans, that the position from which Wnt signals are presented can determine the polarity of both embryonic and postembryonic cells. Furthermore, the position from which a Wnt signal is presented can determine the polarity of Frizzled receptor localization, suggesting that the polarizing effect of Wnt is likely to be direct. These results demonstrate that Wnt proteins can function as positional cues in establishing cell polarity.
Introduction
Wnt signaling pathways play critical roles in establishing cell polarity during normal developmental patterning in C. elegans embryonic and postembryonic cells, developing epithelial cells of Drosophila, and cells undergoing morphogenesis in vertebrate embryos. For this reason, and because loss of cell polarity is an early step in some cancers, there is great interest in understanding exactly how Wnt signals function in cell polarization (Logan and Nusse, 2004; Sancho et al., 2004, Caussinus and Gonzalez, 2005). Much is known about molecular and biochemical mechanisms of Wnt signaling, but some fundamental issues about how Wnt pathways function and how they establish cell polarity remain unresolved (Peifer and Polakis, 2000; Herman, 2002; Vincent and Dubois, 2002; Veeman et al., 2003). Whether Wnt signals function as positional cues during cell polarization is not yet clear (Martinez Arias, 2000; Axelrod and McNeill, 2002; Herman, 2002; Kaltschmidt et al., 2002). While experimental results from many groups have been consistent with Wnt signals functioning as positional cues that can establish polarity, direct tests of this hypothesis by ubiquitous misexpression of Wnts by heat shock expression or mRNA injection into undivided zygotes have often resulted in normal cell polarity in both invertebrate and vertebrate systems (Herman et al., 1995; Whangbo and Kenyon, 1999; Whangbo et al. 2000; Heisenberg et al., 2000). Such results have raised the possibility that Wnt signals might act only as permissive factors in cell polarization (Martinez Arias, 2000; Axelrod and McNeill, 2002; Kaltschmidt et al., 2002).
At the four-cell stage in C. elegans, the P2 cell (referred to here as the signaling cell) is required for two effects in EMS (the responding cell) -- for endoderm development in one daughter of the responding cell and for a specific spindle orientation in the responding cell (see Thorpe et al., 2000 for review). A Wnt signaling protein, MOM-2, from the signaling cell is required for both responses (Fig. 1A) (Rocheleau et al., 1997; Thorpe et al., 1997; Schlesinger et al., 1999). Wnt signaling acts through a TCF/LEF-1 transcription factor, POP-1, to induce endoderm, and acts independently of transcription in spindle orientation (Moon et al., 2002). Endoderm induction is believed to be a cell polarizing interaction because contact between the inducing and responding cells specifically before the responding cell divides is both necessary and sufficient to induce endoderm development (Goldstein, 1995a), and because asymmetries in critical nuclear proteins appear very early, even before the responding cell completes division (Nakamura et al., 2005). The signaling cell expresses a second protein, MES-1, that also plays a role in polarizing the responding cell. MES-1 is a membrane protein that acts in a Src kinase signaling pathway as a redundant signal in endoderm induction and as an essential signal in spindle orientation (Berkowitz and Strome, 2000; Bei et al., 2002). Changing the position of the signaling cell can reorient the polarity of the responding cell (Goldstein, 1995a, 1995b), but it has been unclear whether it is the position of the Wnt signal or MES-1 or both that polarizes EMS.
Figure 1.
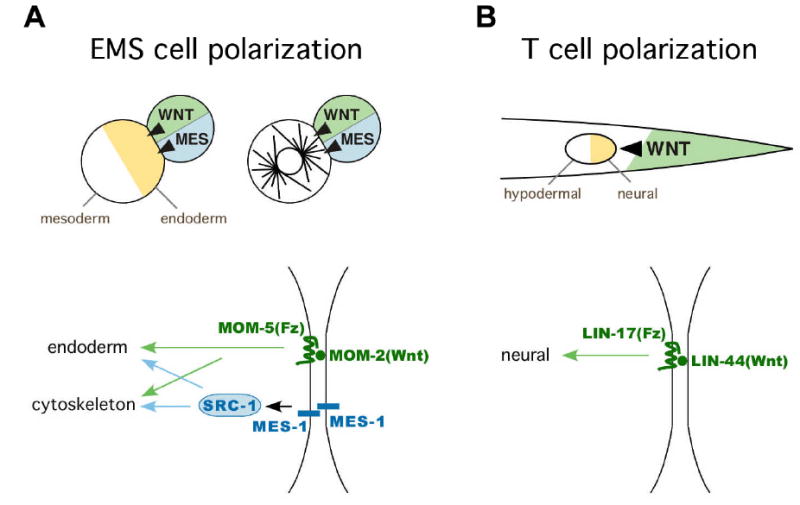
Polarization of the EMS cell and the T cell in C. elegans requires Wnt signals
(A) Wnt and MES-1-dependent pathways play roles in polarization of cell fates (orange and white in EMS cell at left) and cytoskeletal polarity (astral microtubules in EMS cell at right) in EMS at the four cell stage (Bei et al., 2002; Berkowitz and Strome, 2000; Rocheleau et al., 1997; Schlesinger et al., 1999; Thorpe et al., 1997). MES-1: MES-1-dependent signaling pathway (blue), WNT: Wnt pathway (green). MES-1 is required in both the signaling cell and the responding cell for EMS spindle orientation (Bei et al., 2002), although it is not known whether MES-1 proteins in each cell interact homophilically as drawn.
(B) A Wnt signal (LIN-44, green shading and arrowhead) plays a role in polarization of the T cell (orange and white circle).
Wnt signals are also important for the polarity of several postembryonic cells (Herman and Horvitz, 1994; Herman et al., 1995; Whangbo et al., 2000). In both T and V5 cells, the Wnt proteins that regulate polarity (LIN-44 and EGL-20, respectively) are expressed posterior to these cells, in potentially ideal positions to provide positional cues for polarity. It has been shown that myo-2 promoter-driven expression of EGL-20 in the pharynx, far anterior to the V5 cell, can rescue the defects of egl-20 mutants, suggesting that EGL-20 might have only a permissive role in the regulation of polarity (Whangbo et al., 2000). However, we have found that the myo-2 promoter can also drive weak but specific expression of GFP in cells posterior to V5 (data not shown), which might orient V5 polarity in these experiments. Ubiquitous expression of Wnts under heat shock promoters in Wnt-minus backgrounds can rescue the normal polarity of T cells and V5 cells (Herman et al., 1995; Whangbo et al., 2000), but it is not clear whether all cells expressing Wnt proteins under a heat shock promoter also direct these proteins to the cell surface, particularly since Wnt proteins specifically require the Porcupine protein for this to occur (Kadowaki et al., 1996). Hence, these experiments failed to resolve this issue. Drosophila wing cells are polarized by asymmetric Frizzled signaling (Adler, 2002) in a planar polarity pathway that was initially presumed to involve a Wnt signal (Cadigan and Nusse, 1997). No ligand for planar polarity has been identified in Drosophila to date, and knockout of multiple Wnts has not produced a planar polarity phenotype, suggesting that planar polarity in Drosophila may be Wnt-independent (Lawrence et al., 2002; Strutt, 2003). Whether Wnt signals function as positional cues for planar polarity in other organisms, including Xenopus or zebrafish, has not been tested directly (Mlodzik, 2002; Ninomiya et al., 2004).
We have addressed this issue first by using a simplified in vitro system, placing individual embryonic signaling cells of defined genetic backgrounds into specific positions on responding cells and assaying resulting cell polarity. We have also used misexpression experiments in C. elegans to examine this issue, misexpressing a Wnt protein in specific cells neighboring a responding cell on the opposite side from where this Wnt is normally presented.
Results and Discussion
To separate the effects of two partially redundant signals, we placed two signaling cells, one lacking the MOM-2 signal and one lacking the MES-1 signal, on opposite sides of a single responding cell (Fig. 2A). Endoderm, which normally develops from the side that is presented with both signals, was assayed in each resulting cell combination using an endodermal terminal differentiation marker (rhabditin granule development) and cell lineage timing as markers. Endoderm developed in most cases (8/11 cases) indicating that one or both of the signaling cells could rescue endoderm development in the responding cell. To determine whether the Wnt or the MES-1 signal had induced an endodermal lineage to develop in these experiments, cell lineages were traced, and endoderm development was assayed in each resulting cell. In all cases in which endoderm developed, the endodermal cells comprised all of the progeny of one cell -- the daughter of the responding cell that formed on the side where the Wnt-positive (mes-1 minus) cell had been placed (Fig. 2). Analysis of cell cycle timing demonstrated that this cell’s lineage developed with cell cycle timing typical for an endodermal lineage, and the other daughter’s lineage developed with cell cycle timing typical for the daughter on the other side of EMS (Fig. 2). We conclude that the position at which a cell bearing a Wnt signal is placed can determine which side of the responding cell will produce the endodermal lineage.
Figure 2.
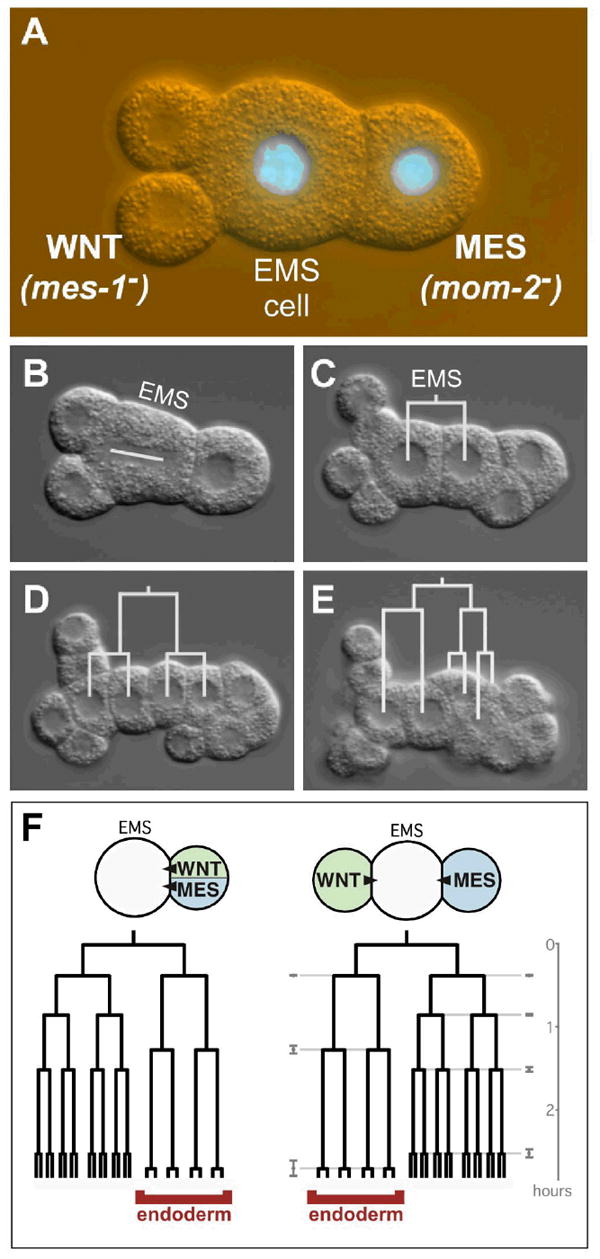
A MOM-2(Wnt) signaling cell can polarize the pattern of cell fates produced by EMS
(A) A mes-1 mutant signaling cell (left) and a mom-2 mutant signaling cell (right) were each isolated and then placed on opposite sides of EMS (for convenience, EMS is also mom-2 minus in this experiment; mom-2 is not required in EMS for endoderm development (Thorpe et al., 1997)). In this picture, the mes-1 mutant signaling cell has divided once, and it has divided more equally than in wild-type, as often occurs in mes-1 mutant signaling cells (Strome et al., 1995). mom-2 mutant cells can be identified by a GFP-histone fusion (green) that was crossed into this strain.
(B) EMS division. White line marks the mitotic spindle.
(C-E) EMS divisions, with cell lineages drawn in white.
(F) Eight out of 11 cases assembled produced endoderm. Typical cell cycle timings are shown from a wild-type cell pair (left) and the average cell cycle timings ± standard deviations (gray) of the eight cases that produced endoderm (right). Endodermal cells are labeled. In the three cases in which endoderm did not develop, lineages of both daughters of EMS resembled the normally uninduced side of EMS (data not shown).
For EMS to produce endoderm, its mitotic spindle must rotate into an orientation that can partition the endoderm-induced side into just one daughter cell (Goldstein, 1995b). Both the Wnt- and MES-1 signaling pathways from the P2 cell are required to induce this mitotic spindle orientation in EMS in cell manipulation experiments (Schlesinger et al., 1999; Bei et al., 2002). This polarization of the responding cell’s cytoskeleton can occur in the absence of new transcription, suggesting that Wnt and MES-1-dependent signaling to the cytoskeleton in this cell is relatively direct (Schlesinger et al., 1999). We determined whether Wnt or MES-1 signaling cells can act as positional cues to polarize the spindle by manipulating cells as described above and assaying the orientation of the responding cell’s mitotic spindle. A cell presenting both Wnt and MES-1 signals was capable of orienting the EMS cell’s mitotic spindle (Fig. 3A), whereas loss of either Wnt or MES-1 signaling resulted in randomized spindle orientations in EMS (Fig. 3B,C), as expected from previous results (Goldstein, 1995b; Schlesinger et al., 1999; Bei et al., 2002). We next tested whether spindle orientation could be aligned in a given axis by placing two signaling cells, one lacking MOM-2 and one lacking MES-1, on opposite sides of the responding cell. We found that these cells could act together to align the mitotic spindle (Fig. 3D), demonstrating that these two signaling pathways need not be presented by the same cell, but are capable of cooperating from opposite sides of a single responding cell.
Figure 3.
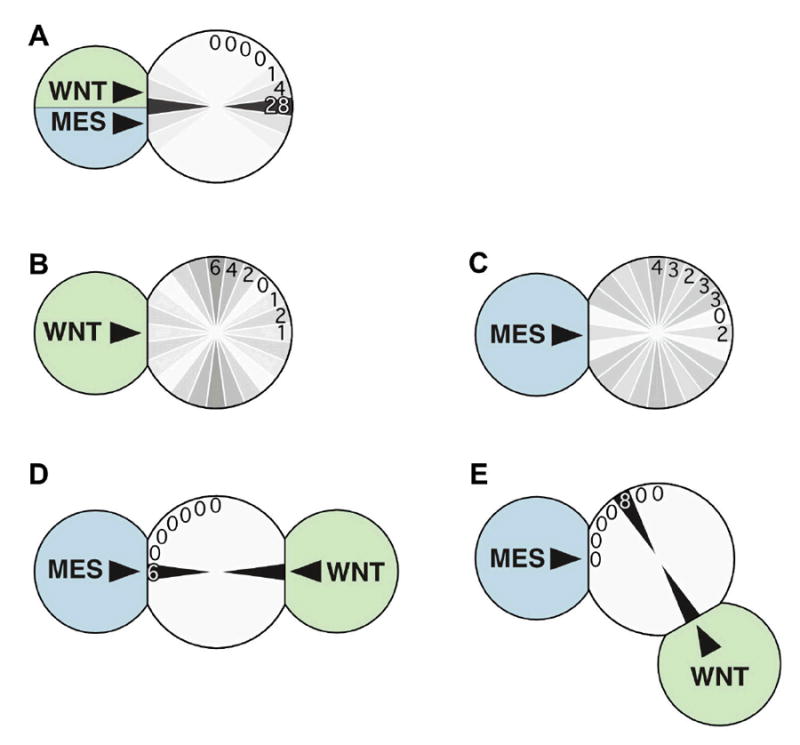
A MOM-2(Wnt) signaling cell can polarize mitotic spindle orientation in EMS when the MES-1 signal is present. Cells bearing signals for Wnt or MES-1 pathways are drawn, and numbers of cases with specific mitotic spindle orientations that resulted are written in one quadrant and diagrammed in all quadrants by gray levels, with darker levels indicating a greater percent of cases with a given spindle orientation.
(A) Wild-type signaling cell orients the responding cell’s spindle.
(B,C) As expected from previous results (Bei et al., 2002; Schlesinger et al., 1999), Wnt- or MES-1-dependent signaling cells alone cannot induce spindle alignment; both signaling and responding cells were mutant in these experiments, although using mutant signaling cells and wild-type responding cells gives similar results (Bei et al., 2002; Schlesinger et al., 1999).
(D) Wnt and MES-1 signaling cells on opposite sides of the responding cell can cooperate to rescue spindle alignment, as seen in Fig. 2b. As a control for the effect of two signaling cells, two mom-2 minus signaling cells were placed similarly on EMS; this resulted in random orientation of the EMS spindle (data not shown).
(E) Wnt and MES-1 signaling cells at roughly orthogonal positions on the responding cell result in spindle alignment toward the Wnt signaling cell.
To determine which cell positions the EMS cell’s mitotic spindle in these experiments, Wnt and MES-1 signaling cells were next presented on the EMS cell from roughly orthogonal positions. We found that the mitotic spindle in EMS consistently oriented in line with the cell presenting the Wnt signal (Fig. 3E). These results reveal that a cell bearing a Wnt signal can act as a positional cue that orients the mitotic spindle, and also that MES-1-dependent signaling can be essential for spindle orientation without providing positional information. We consider it likely that Wnt signaling acts directly on the responding cell, since spindle orientation requires MOM-2(Wnt) only in the signaling cell and MOM-5(Fz) only in the responding cell, and since embryonic transcription is not required for Wnt-induced spindle orientation (Schlesinger et al., 1999).
To determine whether a Wnt pathway can act as an asymmetric cue in a second Wnt-dependent cell polarization event, we expressed a C. elegans Wnt protein, LIN-44, in an ectopic position and examined the effects on asymmetric T cell division. During normal development, the anterior daughter of the T cell produces hypodermal cells, and the posterior daughter produces neural cells (Fig. 1). LIN-44 is normally expressed in cells just posterior to the T cell, and in the absence of this protein, the polarity of T cell division is often reversed (Herman and Horvitz, 1994; Herman et al., 1995). We misexpressed LIN-44 in cells just anterior to the T cell using the egl-5 promoter, which is expressed in cells near the rectum (Wang et al., 1993) (Fig. 4A). In these experiments, we monitored ectopic expression by coexpression of egl-5∷GFP (Fig 4B). This reporter confirmed specific expression just anterior to the T cell in the tail region. We introduced egl-5∷LIN-44 in the absence of endogenous LIN-44 and found that the polarity reversal phenotype was significantly enhanced (Fig. 4A). Moreover, anterior expression of LIN-44 can sometimes reverse the polarity of the division even in wild type animals expressing endogenous LIN-44 (Fig. 4). The Frizzled-like receptor LIN-17 (Sawa et al., 1996) is required for ectopically-expressed LIN-44 to reverse polarity (Fig. 4A). We conclude from these results that a LIN-44-expressing cell can polarize the T cell by signaling through LIN-17. These results demonstrate that as with EMS cell polarization, T cell polarization is controlled by the position from which a Wnt signaling cell is presented to a responding cell.
Figure 4.
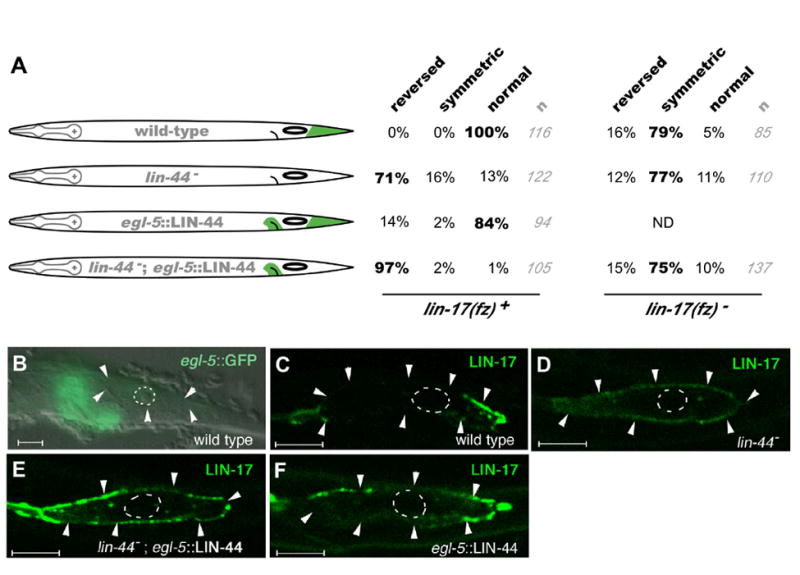
Polarity reversal by anterior expression of LIN-44(Wnt)
(A) Left, schematic drawings of LIN-44 expression (green) relative to T cell position (oval) in strains used in the experiments. Right, percent of animals with reversed, symmetric or normal T cell orientation in the presence or absence of lin-17. The orientation of most T cells for each experiment is highlighted in bold. lin-44; egl-5∷LIN-44 significantly enhances reversals over lin-44 alone (P-value <10-7 by Fisher exact test). The egl-5∷LIN-44 transgene also contains egl-5∷GFP to monitor expression. Number of animals scored is indicated (gray). (B-F) Expression of egl-5∷GFP and LIN-17∷GFP. Cell boundaries are indicated by white arrowheads where they are visible (some cell boundaries are typically not detectable in postembryonic cells). Nuclei are outlined by dotted lines. Scale bars = 5μm.
(B) egl-5∷GFP expression shows that the egl-5 promoter used drives expression in cells just anterior to the T cell.
(C-F) LIN-17(Frizzled) localization in the T cell: LIN-17∷GFP at the cell periphery becomes enriched on the side where LIN-44 is expressed. Genotypes are indicated in white, and expression being followed is indicated in green in each panel. All animals also contain unc-76(e911) rescued by the transgenes containing the unc-76 rescuing plasmid (Bloom and Horvitz, 1997) and scm∷LIN-17∷GFP. Fluorescence was analyzed by confocal microscopy. Anterior is to the left, and ventral is down.
Frizzled receptors have been reported to localize asymmetrically in certain cells in Drosophila and C. elegans (Strutt, 2001; Strutt et al., 2002; Park et al., 2004). However, a relationship between Frizzled localization and the position of a Wnt signal has not been demonstrated. To examine the possibility that Wnt signals may directly influence the localization of Frizzled receptors, we expressed a LIN-17(Frizzled) GFP fusion protein in the seam cells, including the T cell, using a seam cell specific promoter (scm) (Koh and Rothman, 2001). We found that scm∷LIN-17∷GFP could rescue T cell development in lin-17 animals, (8% defective, n=62), suggesting that our GFP fusion is functional and that LIN-17 functions in the T cell and not in the more posterior Wnt signaling cells (Herman et al., 1995). We examined GFP localization and found that LIN-17∷GFP is specifically localized on the posterior side of the T cell, where the T cell contacts LIN-44(Wnt)-producing cells (Fig 4C; 33/33 animals). The asymmetric localization was not observed in lin-44 mutants (Fig 4D; 17/17 animals). Moreover, driving anterior Wnt expression using egl-5∷LIN-44 often caused LIN-17:GFP to localize to the anterior side of the T cell, both in lin-44 mutants (Fig 4E; 13/22 animals) or in the presence of endogenous lin-44 (Fig 4F; 7/35 animals showed the localization on both sides of the T cell). These results suggest that LIN-44 from the signaling cell polarizes the localization of Frizzled receptor in the responding cell. These results show that the position from which a Wnt signal is presented can determine the polarized localization of Frizzled receptor. Since LIN-44(Wnt) functions in the signaling cells, and LIN-17(Frizzled) functions in the responding cell, we suggest that a Wnt signal can act directly to polarize Frizzled localization in a responding cell.
Our results demonstrate that Wnt signaling can orient the polarity of previously unpolarized cells. Other work has implicated Wnts in controlling the orientation of differentiated neuronal cells during axonal guidance (Lyuksyutova et al., 2003; Yoshikawa et al., 2003). Mouse Wnt4 can enhance axonal growth in commissural neurons and can result in extension of axons in the direction of an exogenous Wnt signal (Lyuksyutova et al., 2003). Although Wnt4 knockouts have not been examined similarly, a Frizzled receptor is required for guidance of these axons, suggesting that Wnt-Frizzled signaling normally plays a role in orienting axonal growth (Lyuksyutova et al., 2003). Drosophila Wnt5 also acts as a positional cue for axonal guidance, but in this case, signaling is through the Derailed receptor, an unconventional receptor tyrosine kinase, rather than through Frizzled or Frizzled2, the more well known Wnt receptors (Yoshikawa et al., 2003). The common function of Wnts as asymmetric cues that may orient both unpolarized, undifferentiated cells such as EMS and T in C. elegans and differentiated neurons in Drosophila and mouse may reflect an ancient role for Wnt proteins not just as signals that pattern tissues, but also as positional cues for individual cell polarization.
Experimental Procedures
Strains
C. elegans strains used in this study included N2 (wild-type), SS149 mes-1(bn7), EU855 mom-2(or309)/nT1, lin-44(n1792) and lin-17(n3091). A GFP-histone-labeled mom-2 strain, LP28, was generated by crossing EU855 and AZ212 (unc-119(ed3) ruIs32[unc-119(+) pie-1∷GFP∷H2B]). The egl-5∷LIN-44 construct contains a 9.1kb SalI-RsaI fragment of C08C3 (the egl-5 promoter without the coding sequence) and lin-44 cDNA in pPD49.26 (a gift from A. Fire). The egl-5∷GFP construct contains the same promoter fragment in a derivative of the vector TU#61 (Chalfie et al., 1994). Both egl-5∷LIN-44 and egl-5∷GFP were introduced into lin-44(n1792); unc-76(e911) animals with an unc-76 rescuing plasmid (Bloom and Horvitz, 1997) to obtain an extrachromosomal array osEx101. All of the strains with osEx101 also carried unc-76(e911). egl-5∷GFP was used to monitor where egl-5 drives expression. scm∷LIN-17∷GFP has the scm promoter fragment and full-length LIN-17 cDNA amplified by PCR fused to the Venus derivative of GFP (Nagai et al., 2002). The CRD-deletion variant of LIN-17∷GFP, scm∷ΔCRD-LIN-17∷GFP, was generated by deleting a PvuI-BamHI fragment of scm∷LIN-17∷GFP, removing amino acids I31 to V177. Strains were maintained at 20°C for embryonic experiments and at 22.5°C for postembryonic experiments as described in Brenner (Brenner, 1974), except mes-1(bn7), which was maintained at 15°C and shifted to 25°C 1-2 days before use in experiments. Experiments involving mes-1(bn7) were performed at 25°C. The polarity of the T cell division was scored as described (Sawa et al., 2000).
Cell Manipulations
Cell manipulation experiments were performed by the methods of Edgar (Edgar, 1995). Responding cells were isolated before induction of endoderm or spindle orientation had occurred, and their rotational orientation was randomized as before (Goldstein, 1992, 1995b). Loss of MOM-2 was previously known to not affect MES-1 localization (Berkowitz and Strome, 2000), and MOM-2-dependent signaling has been detected in the absence of MES-1 (Bei et al., 2002; Berkowitz and Strome, 2000), which suggested that each signal could be removed from cells independently before we carried out our cell manipulation experiments. Endoderm development was scored by assaying for birefringent rhabditin granules as described (Goldstein, 1995a) after tracing lineages in 4D recordings (Thomas et al., 1996). Genotypes of isolated cells were identified by isolating cells from each genetic strain separately and by marking one strain in each experiment with a GFP-histone fusion construct. Spindle orientation was assayed by live microscopy and 4D recordings, using the plane of cytokinesis as an easily measurable indicator of the spindle axis.
Acknowledgments
We thank Peter Lawrence, José Casal, Mark Peifer, Roel Nusse, Stefan Hoppler and members of the Goldstein and Sawa labs for discussions and comments on the manuscript. Some strains used in this study were received from the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources. Work from the Goldstein lab was supported by NIH grant R01-GM68966 and a Pew Scholarship in the Biomedical Sciences, and work from the Sawa lab was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology and the Japan Society for the Promotion of Science.
References
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- Axelrod JD, McNeill H. Coupling planar cell polarity signaling to morphogenesis. ScientificWorldJournal. 2002;2:434–454. doi: 10.1100/tsw.2002.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei Y, Hogan J, Berkowitz LA, Soto M, Rocheleau CE, Pang KM, Collins J, Mello CC. SRC-1 and Wnt signaling act together to specify endoderm and to control cleavage orientation in early C. elegans embryos. Dev Cell. 2002;3:113–125. doi: 10.1016/s1534-5807(02)00185-5. [DOI] [PubMed] [Google Scholar]
- Berkowitz LA, Strome S. MES-1, a protein required for unequal divisions of the germline in early C. elegans embryos, resembles receptor tyrosine kinases and is localized to the boundary between the germline and gut cells. Development. 2000;127:4419–4431. doi: 10.1242/dev.127.20.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom L, Horvitz HR. The Caenorhabditis elegans gene unc-76 and its human homologs define a new gene family involved in axonal outgrowth and fasciculation. Proc Natl Acad Sci U S A. 1997;94:3414–3419. doi: 10.1073/pnas.94.7.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Caussinus E, Gonzalez C. Induction of tumor growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nat Genet. 2005;37:1125–1129. doi: 10.1038/ng1632. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Edgar LG. Blastomere culture and analysis. Methods Cell Biol. 1995;48:303–321. doi: 10.1016/s0091-679x(08)61393-x. [DOI] [PubMed] [Google Scholar]
- Goldstein B. Induction of gut in Caenorhabditis elegans embryos. Nature. 1992;357:255–257. doi: 10.1038/357255a0. [DOI] [PubMed] [Google Scholar]
- Goldstein B. An analysis of the response to gut induction in the C. elegans embryo. Development. 1995a;121:1227–1236. doi: 10.1242/dev.121.4.1227. [DOI] [PubMed] [Google Scholar]
- Goldstein B. Cell contacts orient some cell division axes in the Caenorhabditis elegans embryo. J Cell Biol. 1995b;129:1071–1080. doi: 10.1083/jcb.129.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Herman MA. Control of cell polarity by noncanonical Wnt signaling in C. elegans. Semin Cell Dev Biol. 2002;13:233–241. doi: 10.1016/s1084-9521(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Herman MA, Horvitz HR. The Caenorhabditis elegans gene lin-44 controls the polarity of asymmetric cell divisions. Development. 1994;120:1035–1047. doi: 10.1242/dev.120.5.1035. [DOI] [PubMed] [Google Scholar]
- Herman MA, Vassilieva LL, Horvitz HR, Shaw JE, Herman RK. The C. elegans gene lin-44, which controls the polarity of certain asymmetric cell divisions, encodes a Wnt protein and acts cell nonautonomously. Cell. 1995;83:101–110. doi: 10.1016/0092-8674(95)90238-4. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10:3116–3128. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt JA, Lawrence N, Morel V, Balayo T, Fernandez BG, Pelissier A, Jacinto A, Martinez Arias A. Planar polarity and actin dynamics in the epidermis of Drosophila. Nat Cell Biol. 2002;4:937–944. doi: 10.1038/ncb882. [DOI] [PubMed] [Google Scholar]
- Koh K, Rothman JH. ELT-5 and ELT-6 are required continuously to regulate epidermal seam cell differentiation and cell fusion in C. elegans. Development. 2001;128:2867–2880. doi: 10.1242/dev.128.15.2867. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Casal J, Struhl G. Towards a model of the organisation of planar polarity and pattern in the Drosophila abdomen. Development. 2002;129:2749–2760. doi: 10.1242/dev.129.11.2749. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt Signaling Pathway in Development and Disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- Martinez Arias A. The informational content of gradients of Wnt proteins. Sci STKE 2000. 2000:PE1. doi: 10.1126/stke.2000.43.pe1. [DOI] [PubMed] [Google Scholar]
- Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18:564–571. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kim S, Ishidate T, Bei Y, Pang K, Shirayama M, Trzepacz C, Brownell DR, Mello CC. Wnt signaling drives WRM-1/beta-catenin asymmetries in early C. elegans embryos. Genes Dev. 2005;19:1749–1754. doi: 10.1101/gad.1323705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya H, Elinson RP, Winklbauer R. Antero-posterior tissue polarity links mesoderm convergent extension to axial patterning. Nature. 2004;430:364–367. doi: 10.1038/nature02620. [DOI] [PubMed] [Google Scholar]
- Park FD, Tenlen JR, Priess JR. C. elegans MOM-5/frizzled functions in MOM-2/Wnt-independent cell polarity and is localized asymmetrically prior to cell division. Curr Biol. 2004;14:2252–2258. doi: 10.1016/j.cub.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis--a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha YH, Ali M, Priess JR, Mello CC. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell. 1997;90:707–716. doi: 10.1016/s0092-8674(00)80531-0. [DOI] [PubMed] [Google Scholar]
- Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- Sawa H, Kouike H, Okano H. Components of the SWI/SNF complex are required for asymmetric cell division in C. elegans. Mol Cell. 2000;6:617–624. doi: 10.1016/s1097-2765(00)00060-5. [DOI] [PubMed] [Google Scholar]
- Sawa H, Lobel L, Horvitz HR. The Caenorhabditis elegans gene lin-17, which is required for certain asymmetric cell divisions, encodes a putative seven-transmembrane protein similar to the Drosophila frizzled protein. Genes Dev. 1996;10:2189–2197. doi: 10.1101/gad.10.17.2189. [DOI] [PubMed] [Google Scholar]
- Schlesinger A, Shelton CA, Maloof JN, Meneghini M, Bowerman B. Wnt pathway components orient a mitotic spindle in the early Caenorhabditis elegans embryo without requiring gene transcription in the responding cell. Genes Dev. 1999;13:2028–2038. doi: 10.1101/gad.13.15.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S, Martin P, Schierenberg E, Paulsen J. Transformation of the germ line into muscle in mes-1 mutant embryos of C. elegans. Development. 1995;121:2961–2972. doi: 10.1242/dev.121.9.2961. [DOI] [PubMed] [Google Scholar]
- Strutt D. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development. 2003;130:4501–4513. doi: 10.1242/dev.00695. [DOI] [PubMed] [Google Scholar]
- Strutt D, Johnson R, Cooper K, Bray S. Asymmetric localization of frizzled and the determination of notch-dependent cell fate in the Drosophila eye. Curr Biol. 2002;12:813–824. doi: 10.1016/s0960-9822(02)00841-2. [DOI] [PubMed] [Google Scholar]
- Strutt DI. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol Cell. 2001;7:367–375. doi: 10.1016/s1097-2765(01)00184-8. [DOI] [PubMed] [Google Scholar]
- Thomas C, DeVries P, Hardin J, White J. Four-dimensional imaging: computer visualization of 3D movements in living specimens. Science. 1996;273:603–607. doi: 10.1126/science.273.5275.603. [DOI] [PubMed] [Google Scholar]
- Thorpe CJ, Schlesinger A, Carter JC, Bowerman B. Wnt signaling polarizes an early C. elegans blastomere to distinguish endoderm from mesoderm. Cell. 1997;90:695–705. doi: 10.1016/s0092-8674(00)80530-9. [DOI] [PubMed] [Google Scholar]
- Thorpe CJ, Schlesinger A, Bowerman B. Wnt signalling in Caenorhabditis elegans: regulating repressors and polarizing the cytoskeleton. Trends Cell Biol. 2000;10:10–17. doi: 10.1016/s0962-8924(99)01672-4. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Vincent JP, Dubois L. Morphogen transport along epithelia, an integrated trafficking problem. Dev Cell. 2002;3:615–623. doi: 10.1016/s1534-5807(02)00323-4. [DOI] [PubMed] [Google Scholar]
- Wang BB, Muller-Immergluck MM, Austin J, Robinson NT, Chisholm A, Kenyon C. A homeotic gene cluster patterns the anteroposterior body axis of C. elegans. Cell. 1993;74:29–42. doi: 10.1016/0092-8674(93)90292-x. [DOI] [PubMed] [Google Scholar]
- Whangbo J, Kenyon C. A Wnt signaling system that specifies two patterns of cell migration in C. elegans. Mol Cell. 1999;4:851–858. doi: 10.1016/s1097-2765(00)80394-9. [DOI] [PubMed] [Google Scholar]
- Whangbo J, Harris J, Kenyon C. Multiple levels of regulation specify the polarity of an asymmetric cell division in C. elegans. Development. 2000;127:4587–4598. doi: 10.1242/dev.127.21.4587. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S, McKinnon RD, Kokel M, Thomas JB. Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature. 2003;422:583–588. doi: 10.1038/nature01522. [DOI] [PubMed] [Google Scholar]


