Abstract
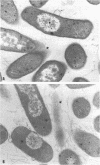
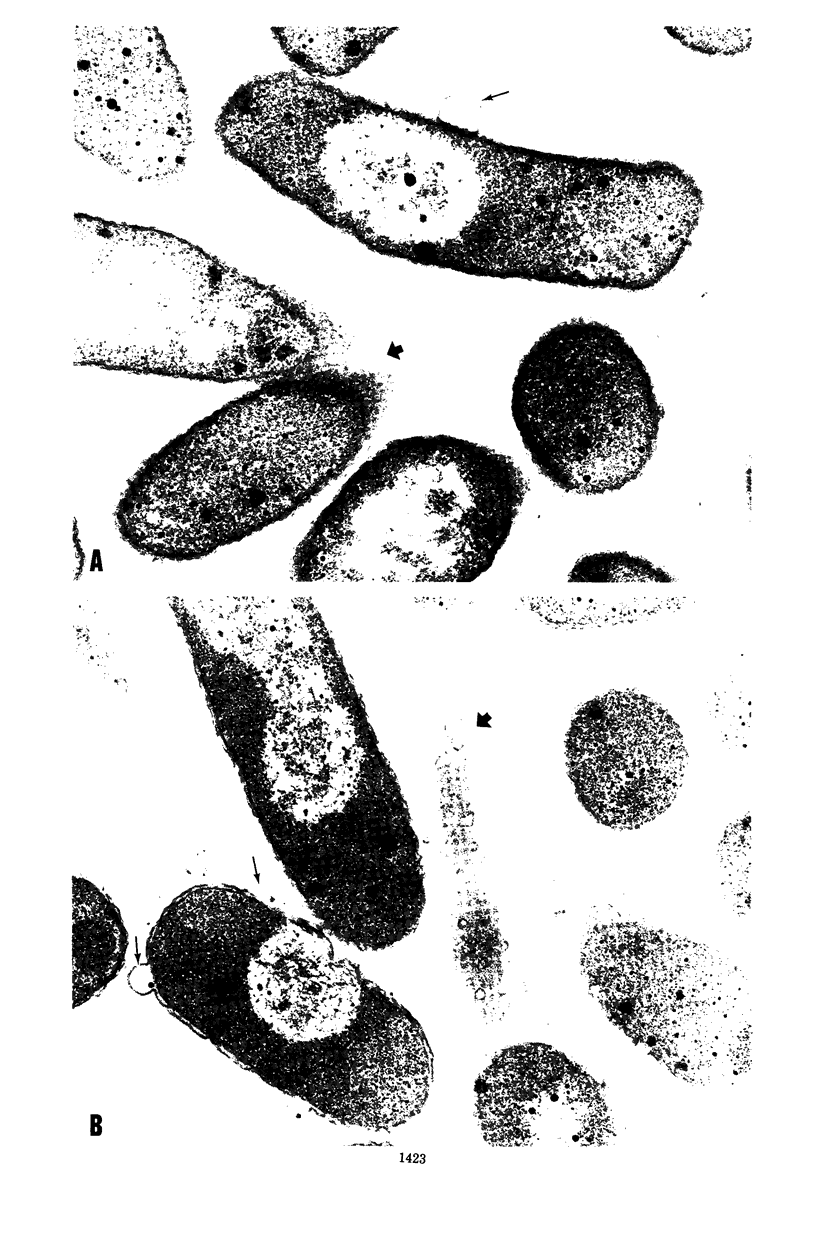
Studies using isogenic transductant strains mlpA+ and mlpA as well as reversion analysis suggested that the physiological consequences of a structural gene mutation in murein lipoprotein include (i) increased sensitivity toward chelating agents ethylenediaminetetraacetic acid and ethyleneglycol-bis (beta-aminoethyl ether)-N,N-tetraacetic acid, (ii) leakage of periplasmic enzyme ribonuclease, (iii) weakened association between the outer membrane and the rigid layer accentuated by Mg2+ starvation, resulting in the formation of outer membrane blebs, and (iv) decreased growth rate in media of low ionic strength or low osmolarity. It is suggested that the bound form of lipoprotein plays an important role in the maintenance of the structural integrity of the outer membrane of the Escherichia coli cell envelope. Other outer membrane components may also contribute to the anchorage of outer membrane to the rigid layer, probably through ionic interactions with divalent cations. Using the phenotype of ribonuclease leakage as an unselected marker in a three-factor cross with P1 transduction, we were able to establish the gene order of man mlpA aroD pps on the E. coli chromosome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Electron microscope study of septum formation in Escherichia coli strains B and B-r during synchronous growth. J Bacteriol. 1974 Sep;119(3):1039–1056. doi: 10.1128/jb.119.3.1039-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. A three-dimensional molecular assembly model of a lipoprotein from the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2396–2400. doi: 10.1073/pnas.71.6.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Lee N., Inouye M., Wu H. C., Suzuki H., Nishimura Y., Iketani H., Hirota Y. Amino acid replacement in a mutant lipoprotein of the Escherichia coli outer membrane. J Bacteriol. 1977 Oct;132(1):308–313. doi: 10.1128/jb.132.1.308-313.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J., Gottfried S., Rothfield L. Leakage of periplasmic enzymes by mutants of Escherichia coli and Salmonella typhimurium: isolation of "periplasmic leaky" mutants. J Bacteriol. 1972 Feb;109(2):520–525. doi: 10.1128/jb.109.2.520-525.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Torti S. V., Park J. T. Lipoprotein of gram-negative bacteria is essential for growth and division. Nature. 1976 Sep 23;263(5575):323–326. doi: 10.1038/263323a0. [DOI] [PubMed] [Google Scholar]
- Venkateswaran P. S., Wu H. C. Isolation and characterization of a phosphonomycin-resistant mutant of Escherichia coli K-12. J Bacteriol. 1972 Jun;110(3):935–944. doi: 10.1128/jb.110.3.935-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand R. A., Shively J. M., Greenawalt J. W. Formation and ultrastructure of extra membranes in Escherichia coli. J Bacteriol. 1970 Apr;102(1):240–249. doi: 10.1128/jb.102.1.240-249.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand R. A., Vinci K. D., Rothfield L. I. Morphogenesis of the bacterial division septum: a new class of septation-defective mutants. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1882–1886. doi: 10.1073/pnas.73.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Hou C., Lin J. J., Yem D. W. Biochemical characterization of a mutant lipoprotein of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1388–1392. doi: 10.1073/pnas.74.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Lin J. J. Escherichia coli mutants altered in murein lipoprotein. J Bacteriol. 1976 Apr;126(1):147–156. doi: 10.1128/jb.126.1.147-156.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Wu T. C. Isolation and characterization of a glucosamine-requiring mutant of Escherichia coli K-12 defective in glucosamine-6-phosphate synthetase. J Bacteriol. 1971 Feb;105(2):455–466. doi: 10.1128/jb.105.2.455-466.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yem D. W., Wu H. C. Genetic characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J Bacteriol. 1977 Sep;131(3):759–764. doi: 10.1128/jb.131.3.759-764.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]