Abstract
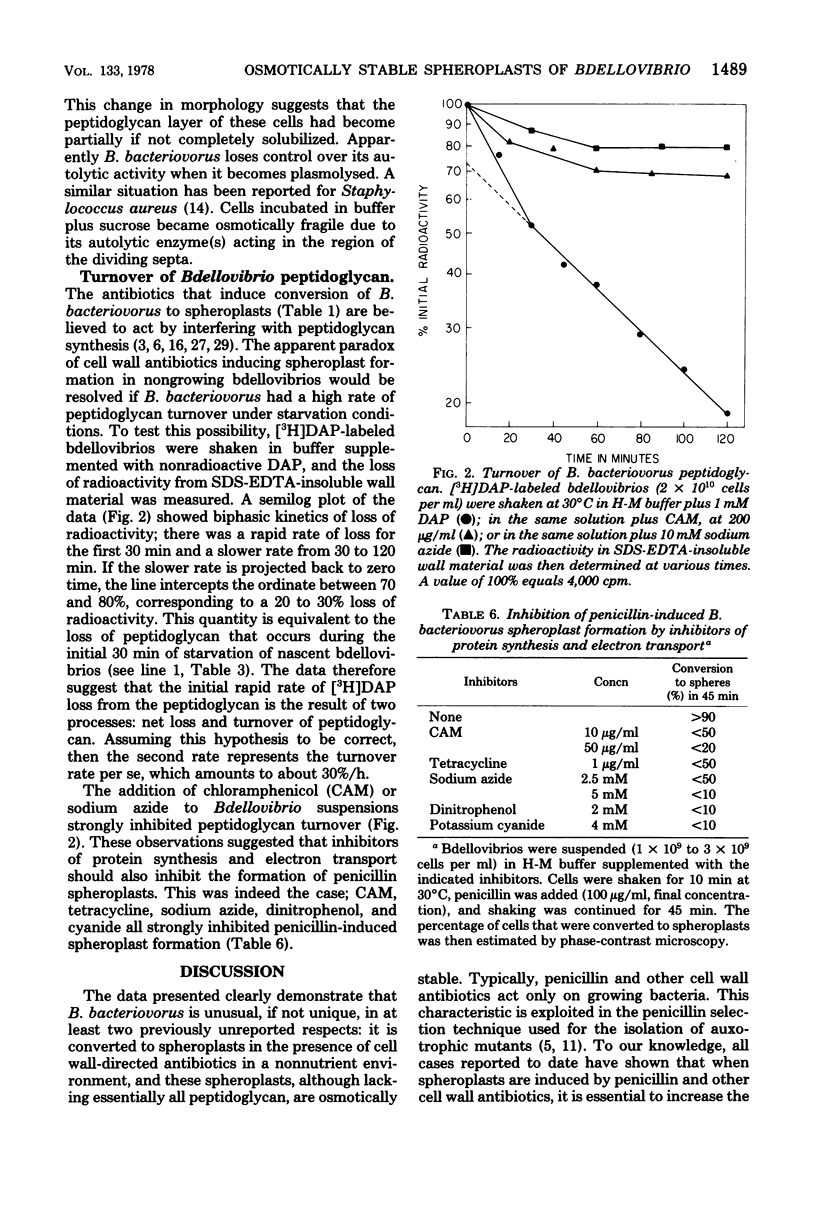
Bdellovibrio peptidoglycan is of typical gram-negative composition. The molar ratios of alanine:glutamic acid:diaminopimelic acid:muramic acid:glucosamine were about 2:1:1:1:1. Nascent, nongrowing Bdellovibrio bacteriovorus 109J were converted from highly motile vibrios to highly motile spheres when shaken in dilute buffer plus penicillin, cephalothin, bacitracin, or D-cycloserine. The spherical forms contained essentially no sedimentable peptidoglycan; i.e., they were spheroplasts. Spheroplasts induced by penicillin, D-cycloserine, and lysozyme were stable in dilute buffer and did not lyse when subjected to osmotic shock. Normal Bdellovibrio suspended in buffer turned over their peptidoglycan at a rate of approximately 30% h during the initial 120 min of starvation. Chloramphenicol and sodium azide strongly inhibited Bdellovibrio peptidoglycan turnover and the induction of spheroplasts by penicillin. The data indicate that nongrowing B. bacteriovorus are sensitive to penicillin and other antibiotics affecting cell walls because of their high rate of peptidoglycan turnover. It is also concluded that an intact peptidoglycan layer is required for maintaining cell shape, but is not required for osmotic stability of B. bacteriovorus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abram D., Davis B. K. Structural properties and features of parasitic Bdellovibrio bacteriovorus. J Bacteriol. 1970 Nov;104(2):948–965. doi: 10.1128/jb.104.2.948-965.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967 Jan;93(1):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Bosch V., Hantke K., Schaller K. Structure and biosynthesis of functionally defined areas of the Escherichia coli outer membrane. Ann N Y Acad Sci. 1974 May 10;235(0):66–82. doi: 10.1111/j.1749-6632.1974.tb43257.x. [DOI] [PubMed] [Google Scholar]
- Hespell R. B., Rosson R. A., Thomashow M. F., Rittenberg S. C. Respiration of Bdellovibrio bacteriovorus strain 109J and its energy substrates for intraperiplasmic growth. J Bacteriol. 1973 Mar;113(3):1280–1288. doi: 10.1128/jb.113.3.1280-1288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., Thomashow M. F., Rittenberg S. C. Changes in cell composition and viability of Bdellovibrio bacteriovorus during starvation. Arch Microbiol. 1974 May 20;97(4):313–327. doi: 10.1007/BF00403070. [DOI] [PubMed] [Google Scholar]
- Kuenen J. G., Rittenberg S. C. Incorporation of long-chain fatty acids of the substrate organism by Bdellovibrio bacteriovorus during intraperiplasmic growth. J Bacteriol. 1975 Mar;121(3):1145–1157. doi: 10.1128/jb.121.3.1145-1157.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J., ST CLAIR J. Protoplasts and L-type growth of Escherichia coli. J Bacteriol. 1958 Feb;75(2):143–160. doi: 10.1128/jb.75.2.143-160.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Perkins H. R., Nieto M. The chemical basis for the action of the vancomycin group of antibiotics. Ann N Y Acad Sci. 1974 May 10;235(0):348–363. doi: 10.1111/j.1749-6632.1974.tb43276.x. [DOI] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative bacteria by lysozyme. Biochim Biophys Acta. 1956 Oct;22(1):189–191. doi: 10.1016/0006-3002(56)90240-2. [DOI] [PubMed] [Google Scholar]
- Rittenberg S. C., Hespell R. B. Energy efficiency of intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1158–1165. doi: 10.1128/jb.121.3.1158-1165.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C., Langley D. Utilization of nucleoside monophosphates per Se for intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1137–1144. doi: 10.1128/jb.121.3.1137-1144.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C. Nonidentity of Bdellovibrio bacteriovorus strains 109D and 109J. J Bacteriol. 1972 Jan;109(1):432–433. doi: 10.1128/jb.109.1.432-433.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C., Shilo M. Early host damage in the infection cycle of Bdellovibrio bacteriovorus. J Bacteriol. 1970 Apr;102(1):149–160. doi: 10.1128/jb.102.1.149-160.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Forsberg C. W. Role of autolysins in the killing of bacteria by some bactericidal antibiotics. J Bacteriol. 1971 Dec;108(3):1235–1243. doi: 10.1128/jb.108.3.1235-1243.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayare M., Daneo-Moore L., Shockman G. D. Influence of macromolecular biosynthesis on cellular autolysis in Streptococcus faecalis. J Bacteriol. 1972 Oct;112(1):337–344. doi: 10.1128/jb.112.1.337-344.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo M., Bruff B. Lysis of Gram-negative bacteria by host-independent ectoparasitic Bdellovibrio bacteriovorus isolates. J Gen Microbiol. 1965 Sep;40(3):317–328. doi: 10.1099/00221287-40-3-317. [DOI] [PubMed] [Google Scholar]
- Shilo M. Morphological and physiological aspects of the interaction of Bdellovibrio with host bacteria. Curr Top Microbiol Immunol. 1969;50:174–204. doi: 10.1007/978-3-642-46169-9_6. [DOI] [PubMed] [Google Scholar]
- Starr M. P., Huang J. C. Physiology of the bdellovibrios. Adv Microb Physiol. 1972;8:215–261. doi: 10.1016/s0065-2911(08)60191-5. [DOI] [PubMed] [Google Scholar]
- Storm D. R. Mechanism of bacitracin action: a specific lipid-peptide interaction. Ann N Y Acad Sci. 1974 May 10;235(0):387–398. doi: 10.1111/j.1749-6632.1974.tb43278.x. [DOI] [PubMed] [Google Scholar]
- Tinelli R., Shilo M., Laurent M., Ghuysen J. M. De le présence d'un glycopeptide dans la paroi de Bdellovibrio bacterioviorus. C R Acad Sci Hebd Seances Acad Sci D. 1970 May 25;270(21):2600–2602. [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- VAITUZIS Z., DOETSCH R. N. FLAGELLA OF SALMONELLA TYPHIMURIUM SPHEROPLASTS. J Bacteriol. 1965 Jun;89:1586–1593. doi: 10.1128/jb.89.6.1586-1593.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon M., Shil M. Interacton of Bdellovibrio bacteriovorus and host bacteria. I. Kinetic studies of attachment and invasion of Escherichia coli B by Bdellovibrio bacteriovorus. J Bacteriol. 1968 Mar;95(3):744–753. doi: 10.1128/jb.95.3.744-753.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C. The isolation of protoplasts from Bacillus megaterium by controlled treatment with lysozyme. J Bacteriol. 1953 Dec;66(6):688–695. doi: 10.1128/jb.66.6.688-695.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]




