Abstract
The expression of brain-derived neurotrophic factor (BDNF) mRNA is increased significantly within the high vocal center (HVc) of male but not female zebra finches from posthatching day 30–35 on. The population of HVc cells expressing BDNF mRNA included 35% of the neurons projecting to the nucleus robustus of the archistriatum (RA). In the RA and in RA-projecting neurons of the lateral portion of the magnocellular nucleus of the anterior neostriatum, BDNF mRNA was expressed at very low levels in both sexes. The BDNF-receptor trkB mRNA was expressed in the RA, in RA-projecting neurons of lateral portion of the magnocellular nucleus of the anterior neostriatum, and in the HVc, except in most of its RA-projecting neurons. Premature stimulation and an inhibitory effect on the normal increase of the BDNF mRNA expression in juvenile males occurred after treatments with 17β-estradiol and the aromatase inhibitor fadrozole, respectively. The up-regulation of the BDNF expression in the HVc could be a mechanism by which estrogen triggers the differentiation of cells within and connected to the HVc of male zebra finches.
In the zebra finch, forebrain circuits are the substrates for a learned motor program leading to singing in males, exclusively. These circuits are sexually dimorphic and include song control nuclei such as high vocal center (HVc), nucleus robustus of the archistriatum (RA), and lateral portion of the magnocellular nucleus of the anterior neostriatum (lMAN) (1, 2). In juvenile males the development of the song and song control nuclei is known to be affected by androgens and estrogens (3–9). In females the song control system can be masculinized by the treatment of hatchlings with pharmacological doses of estradiol (10). However, within the song control system, the expression of the estrogen receptor is confined to cells located in the ventral caudomedial portion of the HVc (11–15). Therefore, estrogen was assumed to indirectly affect cells of the song control nuclei that do not express the estrogen receptor by inducing the production of paracrine and retro- and/or anterogradely acting factors (13).
It is well known that members of the neurotrophin gene family, such as brain-derived neurotrophic factor (BDNF), affect the differentiation and functions of central neurons (16–19), and numerous studies have provided evidence for several modes of actions of BDNF within the central nervous system (20). Furthermore, the expression of neurotrophins and their receptors can be triggered by sex hormones (21). Estrogen treatments result in an enhanced BDNF mRNA expression in the cerebral cortex and the olfactory bulb of ovarectomized rat (22). After testosterone treatment, the HVc of adult female canaries was shown recently to exhibit an increased immunostaining for BDNF (23).
We investigated whether the expression pattern of BDNF mRNA is consistent with a role of BDNF as a mediator of estrogenic effects on the development of the song control system of the zebra finch. The expression patterns of BDNF and BDNF-receptor trkB mRNA in the juvenile HVc, RA, and lMAN were obtained by in situ hybridizations with cRNA probes for zebra finch BDNF and trkB mRNA. In males, we found BDNF mRNA expression to be increased dramatically in the HVc from posthatching day (P)30–35 on, whereas in all juveniles, RA cells and targets of RA neurons expressed the BDNF mRNA at a very low level. In the male HVc, BDNF mRNA was expressed in a subpopulation of RA-projecting neurons, and in RA cells, the expression of trkB mRNA was clearly detectable. The BDNF mRNA expression in the HVc of juvenile males was found to be prematurely stimulated by exogenous estrogen and prevented by an inhibitor of aromatase, which is the key enzyme to convert androgens into estrogens. These data suggest a mechanism by which estrogens could regulate indirectly the differentiation of cells within the juvenile male song control system.
MATERIALS AND METHODS
Animals and Experimental Groups.
Zebra finches (Taeniopygia guttata) were reared in our breeding colonies, killed by an overdose of isofluran, and examined for the gonadal sex. Brains were removed and frozen over liquid nitrogen. The chilled brains were subjected to in situ hybridization procedures. Androgen receptor (AR), BDNF, and trkB mRNA expression in the HVc, lMAN, and RA were analyzed in either sex at P20–25 and P30–35 (n = 6 and 11, respectively, for each sex). For retrograde labeling of RA-projecting neurons in the male, HVc and lMAN birds anesthetized by isofluran received stereotaxical microinjections (0.1 μl) of 20% lysine-fixable dextran-Texas Red (Molecular Probes) in PBS. Birds (HVc, three males at P35; lMAN, six males at P20–35) were sacrificed 2 days after the injections. 17β-Estradiol (70 μg; Sigma) was administered in directly implanted silastic pellets as described by Gurney (24). Short-term treatments of juvenile males (P10, n = 2; P15, n = 3; P20–25, n = 6) and females (P19–23, n = 5) with 17β-estradiol were performed for 24–48 hr. Additional females were treated with 17β-estradiol at P16–21 for 4–6 days (n = 4). For long-term treatments of females sacrificed at P33–45 (n = 4), 17β-estradiol pellets were implanted at P5–10.
To ascertain whether endogenous estrogens trigger the BDNF mRNA expression in the song control system we treated juvenile males with the aromatase inhibitor fadrozole (CIBA–Geigy). Fadrozole is well known to block specifically the aromatase activity in the zebra finch (25). Pellets containing 125 μg fadrozole were implanted s.c. into P28 males (n = 6). These birds were sacrificed at P35.
In Situ Hybridization.
Cloning and hybridization procedures for AR probes are described elsewhere (26, 27). Probes for the detection of AR mRNA were used to determine the position of the HVc and RA, which are regions showing a high AR mRNA expression level (Figs. 1 and 2) in juveniles.
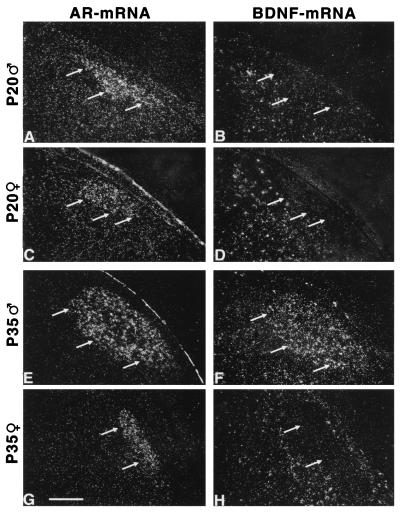
Figure 1.
Sex-specific increase of the BDNF mRNA expression in the HVc. Depicted are dark-field photomicrographs of the caudal neostriatum of a P20 male (A and B), a P20 female (C and D), a P35 male (E and F), and a P35 female (G and H) zebra finch. In A, C, E, and G, in situ hybridizations for the androgen receptor (AR) mRNA are shown. The HVc is clearly defined by a high expression level of AR mRNA in both males and females. Arrows indicate the ventral border of the HVc. In B, D, F, and H, in situ hybridizations for BDNF mRNA are shown. These sections are adjacent to the sections hybridized with the probe for AR mRNA. HVc cells express BDNF mRNA in the P35 male (F) but not in the P35 female (H) or P20 birds (B and D). (Bar = 50 μm.)
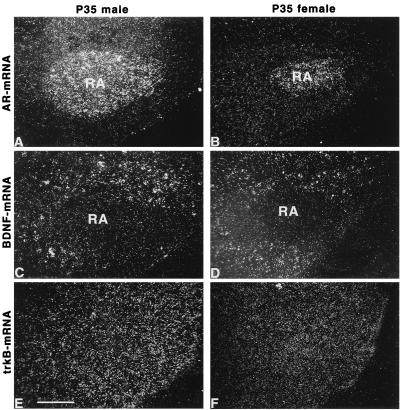
Figure 2.
RA cells in either sex express trkB but not BDNF mRNA. Shown are dark-field photomicrographs of adjacent sections obtained from the archistriatum of P35 zebra finches. The nucleus is defined by its expression of AR mRNA (A and B). In situ hybridizations for BDNF (C and D) and trkB mRNA (E and F) are shown on adjacent sections for P35 male (A, C, and E) and female (B, D, and F) zebra finches. RA cells do not express BDNF mRNA (C and D) in either sex. However, the BDNF mRNA expression could be observed in the surroundings of the RA (C and D). Expression of trkB mRNA was detectable throughout the archistriatum including the RA (E and F). (Bar = 50 μm.)
Probes for zebra finch BDNF and trkB mRNA were cloned by standard PCR techniques. Complementary DNAs were synthesized by using mRNA isolated from the brains of adult zebra finches by Micro-Fast Track kit (Invitrogen), and PCR amplifications were performed with the following primer sets: for BDNF, the 5′ primer was 5′-(CT)(AG)GTTGCATGAA(AG)GCTGC(CG)C-3′ and the 3′ primer was 5′-T(GA)ACATGTTTGC(AG)GCATCC-3′; for trkB, the 5′ primer was 5′-GACGATG A(CT)TCTGCCAGTC-3′ and the 3′ primer was 5′-CCAGGATCTTGTCCTGCTC-3′. We obtained a 290-bp fragment that is 91% homologous with the prepro-part of chicken BDNF (M83377) and a 297-bp fragment showing 93% homology with a sequence coding for a part of the extracellular domain and the transmembrane domain of chicken trkB (X77251). The fragments were cloned into the SmaI site of pGEM 7Zf(+), and 35S-labeled cRNA probes were generated.
Brain sections were hybridized at 55°C with sense or antisense probes in 10 mM Tris⋅HCl (pH 7.5) containing 600 mM NaCl/50% formamide/0.02% Ficoll/0.02% BSA/0.02% polyvinylpyrrolidone/1 mM EDTA/0.01% salmon testicular DNA/0.05% total yeast RNA/0.005% yeast t-RNA/10% dextran sulfate/0.1% SDS/0.1% sodium thiosulfate/100 mM DTT. After hybridization, slides were treated for 30 min with RNase-A (20 μg/ml) in RNase buffer (10 mM Tris⋅HCl, pH 8.0/0.5 M NaCl/1.0 mM EDTA) and subsequently washed in the same buffer for 30 min. The slides then were washed in 2× standard SSC for 30 min at 50°C, 0.2× SSC for 30 min at 55°C, and, finally, in 0.2× SSC for 30 min at 60°C. Washed slides were dehydrated and dipped into Kodak NTB-2 nuclear track emulsion diluted 4:5 with 0.1% Aerosol 22 (Sigma) and exposed at room temperature for 5–8 days.
On sections hybridized with sense probes no specific labeling occurred. Therefore, only photomicrographs of in situ hybridizations with antisense probes are presented here. The trkB mRNA probes we used for in situ hybridization would be expected to detect the mRNA for both the tyrosine kinase isoform as well as the truncated trkB receptors, which lack the intracellular kinase catalytic region. However, RA neurons have been shown to be responsive to BDNF (28). Furthermore, truncated trkB receptors also have been found to mediate BDNF-induced signal transduction in vitro (29).
For quantification of in situ results we first analyzed whether the labeling obtained with the antisense probe was different from the sense probe of the same area and animal, i.e., was not random. For this purpose, images of autoradiograms were scanned into an image analysis system (Imatec, Munich). The outlines of HVc or other brain areas were defined because of the expression of AR mRNA (see Figs. 1, 2, and 4). Using a subsampling algorithm, the number of grains within randomly sampled, cell-sized areas in the study areas were determined. This subsampling algorithm is independent of any background criteria and allows the quantification over a large number (n = 1,000) of cell-sized areas per brain nucleus. In brief, the number of silver grains over 10 cell-sized areas randomly selected with a computer program in the HVc was summed up. This procedure was repeated 20 times for each of five HVc sections per animal. The resulting distribution curves of numbers of silver grains over cell-sized areas (see Fig. 5) obtained with sense and antisense probes were compared by using the Kolmogorow–Smirnow two-sample test (KS). The same test also was used to compare the distribution curves of labeling intensity between the experimental groups.
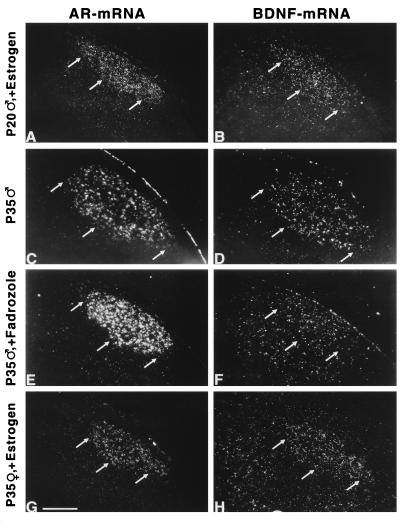
Figure 4.
Estrogen-dependent BDNF mRNA expression in the zebra finch HVc. Depicted are dark-field photomicrographs of the caudal neostriatum of a short-term (24-hr), estrogen-treated P20 male (A and B), a P35 male (C and D), a P35 male treated with the aromatase inhibitor fadrozole for 7 days (E and F), and a P33 female that received a 17β-estradiol implant at P10 (G and H). In A, C, E, and G, the HVc is defined by a high level of AR mRNA expression. In B, D, F, and H, the expression of BDNF mRNA in sections adjacent to A, C, E, and G is shown. Arrows indicate the ventral border of the HVc. For the estrogen treatments, 17β-estradiol-filled implants (70 μg) were used. For treatment with an aromatase inhibitor, pellets containing 125 μg fadrozole were implanted at P28. An estrogen treatment for 24 hr was sufficient to induce the BDNF mRNA expression in young male HVc. The physiological increase of the BDNF mRNA expression in the male HVc was inhibited after treatment with fadrozole. The grain density over the HVc of males treated with fadrozole was similar to the grain density over surrounding areas and not different from controls after hybridization with sense probes for BDNF mRNA. In females, only early estrogen treatment resulted in a BDNF mRNA expression in HVc cells around P30. (Bar = 50 μm.)
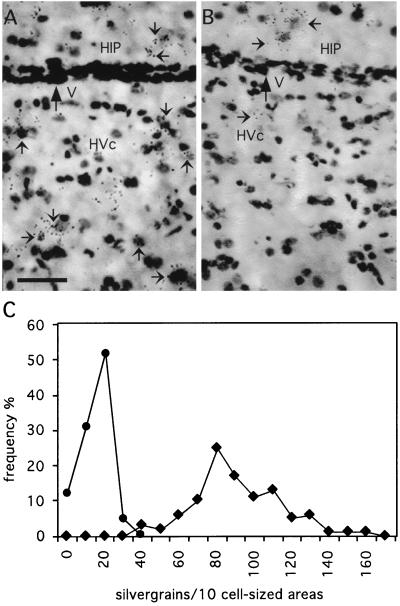
Figure 5.
Higher-power photomicrographs of the BDNF mRNA expression in the HVc and hippocampus (HIP) of an estrogen-treated, 24-day-old (A) and an estrogen-formation-blocked (fadrozole-treated), 35-day-old (B) male zebra finch. Shown are parts of the HIP and HVc in bright-field photomicrographs of the Nissl-stained autoradiograms. Small arrows indicate some of the labeled cells. Note that the BDNF mRNA is expressed in the HIP of males with high (A) and low (B) estrogen levels. In contrast, BDNF mRNA expression in the HVc is estrogen-dependent, i.e., high in estrogen-treated animals and low in animals with blocked estrogen formation. V, lateral ventricle. (Bar = 20 μm.) In C, the number of silver grains over cell-sized areas in the HVc of the estrogen-treated (solid squares) and fadrozole-treated (solid circles) animals of A and B are shown. The numbers of silver grains in the HVc over 10 cell-sized areas randomly selected with a computer program were summed up. This procedure was repeated 20 times for each of five HVc sections per animal. The HVc of the estrogen-treated animal contains significantly more silver grains compared with the fadrozole-treated animal.
We analyzed next the percentage of labeled cells in an area by using the 99% Poisson criterion (30). The total number of cells within a certain brain area was obtained by using the dissector method (31). With the assistance of the image analysis system, the cellular expression of AR, BDNF, and trkB mRNA was analyzed under high-power magnification (×1,000) in five sections per brain nucleus and animal (see Table 1). The percentage of AR, BDNF, or trkB mRNA-expressing cells among all cells of an area then was calculated based on the total number of cells within the same area.
Table 1.
Effect of age and estrogen treatment on the AR and BDNF mRNA expression level in song control nuclei of males
| P20–25 (n = 6)
|
P30–35 (n = 6)
|
P20–25 + E2 (n = 6)
|
||||
|---|---|---|---|---|---|---|
| AR | BDNF | AR | BDNF | AR | BDNF | |
| HVc | 35.4 ± 3.3 | 1.5 ± 0.3 | 29.0 ± 4.5 | 23.0 ± 4.2 | 27.5 ± 1.7 | 19.5 ± 3.8 |
| IMAN | 28.6 ± 2.9 | 0.9 ± 0.2 | 33.0 ± 7.1 | 1.0 ± 0.3 | 26.2 ± 4.1 | 1.2 ± 0.4 |
| RA | 8.1 ± 3.5 | 1.1 ± 0.5 | 12.5 ± 3.5 | 1.8 ± 0.7 | 9.8 ± 2.6 | 1.4 ± 0.3 |
| nXIIts | 31.0 ± 2.7 | 0.2 ± 0.1 | 35.0 ± 4.2 | 0.2 ± 0.1 | 24.3 ± 1.1 | 0.1 ± 0.1 |
| Anterior neostriatum | 1.6 ± 0.7 | 12.8 ± 1.2 | 1.0 ± 0.3 | 15.3 ± 2.8 | 0.8 ± 0.2 | 0.1 ± 0.1 |
The expression level in a brain region is indicated by the percentage (mean ± SD) of the number of labeled cells by using the 99% Poisson criterion (30) after in situ hybridization for AR or BDNF mRNA. Values around 1% or less are in the range of the expected number of false positives by using the 99% Poisson criterion. The expression level of BDNF mRNA in the HVc is significantly higher in P30–35 compared with P20–25 males and is increased significantly at P20–25 after treatment with estrogen (E2) (t test; P = 0.001). Age or estrogen treatment had no significant effect on the BDNF mRNA expression level either in IMAN and RA or in the anterior neostriatum. The expression level of AR mRNA was not affected by age or estrogen treatment in the areas investigated. nXIIts, tracheosyringeal part of the hypoglossal nucleus.
To determine the percentage of BDNF mRNA-expressing cells among retrogradely labeled HVc neurons, for each bird 100 neurons were analyzed by using the 99% Poisson criterion (30). The percentages of BDNF mRNA-expressing cells were compared between experimental groups by using Student’s t test.
RESULTS
The Expression Pattern of BDNF and trkB mRNA in Juvenile Song Control Nuclei.
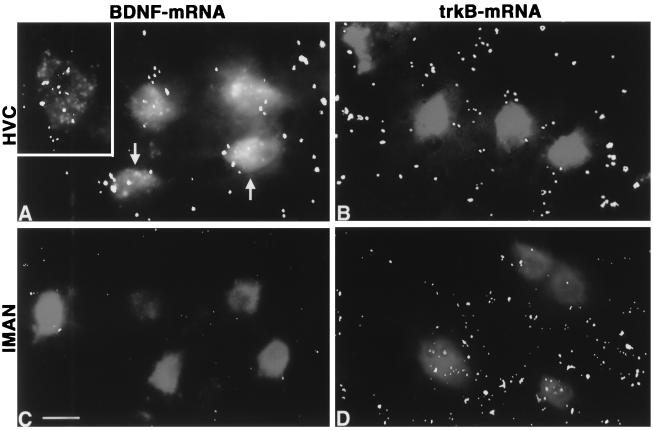
In juvenile zebra finches the forebrain song control nuclei HVc, lMAN, and RA can be distinguished unambiguously by a high AR mRNA expression level (Fig. 1 A, C, E, and G and ref. 32). On forebrain sections of 20-day-old birds, BDNF mRNA-expressing cells could be observed ventral to HVc but not in HVc (Fig. 1 B and D). Within the HVc, the BDNF mRNA expression was found to be different from a random distribution in males (KS; P < 0.0001) but not in females starting at P30–35 (Fig. 1). Thus, BDNF mRNA was found to be expressed in 23 ± 4.2% of male HVc cells at P35. In contrast, the expression level of AR mRNA in the HVc did not undergo developmental changes (Table 1).
In both the male and female RA, the expression of BDNF mRNA was undetectable by using the 99% Poisson criterion (Table 1), whereas cells expressing BDNF mRNA were found in the surroundings of the nucleus (Fig. 2 C and D). In the lMAN, BDNF mRNA was expressed at a low level in juveniles of either sex compared with the surrounding anterior neostriatum (Table 1). To determine whether RA-projecting neurons are potential sources of BDNF anterogradely acting on RA cells, we investigated the expression of BDNF mRNA in these neurons after retrograde labeling. In P30–35 males, 27 ± 3.5% of the RA-projecting neurons in the HVc exhibited BDNF mRNA expression (Fig. 3A). In contrast, RA-projecting neurons of the lMAN did not express BDNF mRNA in males (Fig. 3C) and females. The labeling of these lMAN neurons was similar for sense and antisense BDNF probes. In regions that are known to be innervated by the RA, including the tracheosyringeal part of the hypoglossal nucleus (nXIIts) (Table 1), the nucleus retroambigualis, the rostroventral respiratory group of the medulla, and the dorsomedial nucleus (33), BDNF mRNA was undetectable. This suggests that HVc cells are a dominant source for the production of BDNF in the song control system. Furthermore, BDNF acting on RA cells seems to be provided predominantly by HVc neurons.
Figure 3.
BDNF and BDNF-receptor trkB mRNA expression in RA-projecting neurons of the HVc and lMAN. Presented are computer-assisted overlays of fluorescence photomicrographs of RA-projecting neurons (gray) with dark-field photomicrographs of in situ hybridizations for BDNF (A and C) and trkB (B and D) mRNA (large, white spots are composed of several silver grains) obtained from the same areas in the HVc (A and B) and the lMAN (C and D) of P35 male zebra finches. RA-projecting neurons were labeled by stereotaxical pressure injections of dextran–Texas Red into the RA of P33 birds. The insert in A shows an accumulation of grains over the soma of an RA-projecting neuron in the HVc. Arrows in A mark RA-projecting neurons in the HVc that express BDNF mRNA. In the HVc, BDNF and trk mRNA were expressed in approximately 27 and 2% of the RA-projecting neurons, respectively. In contrast, RA-projecting neurons of the lMAN were found to express trkB but not BDNF mRNA. (Bar = 10 μm.)
Target cells of BDNF were identified by in situ hybridization for the BDNF receptor trkB. TrkB mRNA-expressing cells were detectable in the RA (Fig. 2 E and F), HVc, and lMAN of either sex, confirming the trkB-immunopositive staining documented for somata and fibers in the RA (28). Thus, RA cells are potential targets of BDNF. The expression of trkB mRNA was found in 45–62% of RA-projecting neurons in the juvenile male lMAN whereas 2% of the analyzed neurons projecting from the HVc to RA showed a trkB mRNA expression (Fig. 3 B and D).
Estrogen-Dependent BDNF mRNA Expression in the Juvenile HVc.
The expression of BDNF mRNA could be induced within 24 hr in the HVc of males by systemic estrogen treatments at P15 and P20–25 (Fig. 4B) but not at P10. The level of the BDNF mRNA expression within the HVc was found to be different between untreated and estrogen-treated males at P20–25 (KS; P < 0.0001) and similar in estrogen-treated P20–25 males and normal P30–35 males by using the subsampling quantification. This also can be seen in the significantly (t test; P < 0.001 for each comparison) higher percentage of BDNF mRNA-expressing HVc cells in the estrogen-treated P20–25 males and the normal P30–35 males compared with untreated P20–25 males (Table 1). The estrogen-inducible increase of the BDNF mRNA expression in the zebra finch brain was area-specific. Estrogen treatments did not result in an enhanced BDNF mRNA expression level in other song control nuclei such as RA and lMAN (Table 1).
The estrogen-dependent stimulation of the BDNF mRNA expression in the male HVc turned out to be transient when the birds were analyzed several days after removal of the estrogen implants. For example, in a P24 male the HVc did not show any BDNF mRNA expression after estrogen treatment for 24 hr starting at P20. Thus, 3 days after removing the estrogen implant, the level of estrogen available to its target cells seems reduced to a concentration at which the expression of BDNF mRNA within the HVc is not affected. This indicates that premature expression of BDNF mRNA in young male HVc depends on a permanently elevated estrogen level. The physiological up-regulation of the BDNF mRNA expression in the P30–35 male HVc was blocked after implantation of pellets containing the aromatase inhibitor fadrozole (Fig. 4F). In these animals, the BDNF mRNA expression in the HVc was found to be significantly different from normal P30–35 males (KS; P < 0.0001) and from estrogen-treated P20–25 (KS; P < 0.0001) males (Fig. 5) by using the subsampling quantification. Blocking of the aromatase reduced the percentage of BDNF mRNA-labeled HVc cells by 80–90% but did not affect the BDNF mRNA expression in the anatomically nearby-located hippocampus (Fig. 5), which excludes procedural problems with the in situ hybridization procedure as a reason for this result.
In contrast to males, juvenile females treated with estrogen for 24–48 hr or for 4–6 days did not exhibit any BDNF mRNA expression in the HVc. However, the BDNF mRNA expression was detectable at P35 in the female HVc when 17β-estradiol pellets were implanted at P5–10 (Fig. 4H). The percentage of BDNF mRNA-expressing cells in these estrogen-treated females was similar to that in males (t test; P = 0.6). Thus, in such early masculinized females, the BDNF mRNA expression occurred in the HVc at the same developmental time point as in untreated males.
DISCUSSION
Estrogen-Dependent Increase of the BDNF mRNA Expression in the Juvenile HVc.
In the male HVc, BDNF mRNA starts to be expressed at a high level around P30. However, a premature expression of BDNF mRNA was inducible in the male HVc after short-term estrogen treatment as early as P15. The transience of this effect revealed that the BDNF mRNA expression in the HVC of young males remains permanently dependent on an increased estrogen level. Furthermore, it suggests that under physiological conditions, elevated sex hormone levels are required for an increase of BDNF mRNA expression in the developing male HVc around P30. Consistent with this suggestion, increased plasma levels of estradiol were reported for male zebra finches around P30–50 (34, 35). The inhibition of the normally occurring increase of the BDNF mRNA expression level in the male HVc after treatment with the aromatase inhibitor fadrozole supports the view that this increase is estrogen-dependent.
The mechanism by which estrogens stimulate the BDNF mRNA expression in the male HVc is still unclear. Whereas most estrogen receptors are located in the ventral caudomedial portion of the HVc (11–15), BDNF mRNA was expressed within the entire HVc. This suggests that in most parts of the HVc the estrogen-dependent expression of BDNF mRNA is not mediated directly by genomic mechanisms involving the estrogen receptor.
In females, the plasma level of estradiol also was reported to increase around P30 (35). Why do they not respond to this surge in estrogen with the expression of BDNF mRNA in the HVc? In females, BDNF mRNA was expressed by HVc cells at P35 only after early estrogen treatments. Thus, it is tempting to speculate that in female hatchlings estrogens prime prospective HVc cells to respond to increasing levels of estrogen during the phase of song learning at P30–35. It is well established that early estrogen treatments masculinize different aspects of singing and have effects on the morphology of the song control system (1, 2, 10). However, the sex difference of juvenile HVc cells to respond to estrogens with an increased BDNF mRNA expression level might be controlled independent of gonadal hormones. High doses of exogenous estrogen might trigger cellular signal transduction pathways in female hatchlings that normally are induced exclusively in males (2, 10).
BDNF Produced in the HVc Could Mediate Estrogen Effects on Other Nuclei of the Song Control System.
The data presented here together with measurements of the BDNF protein level in the song control system (36) clearly demonstrate that the HVc is the major site of BDNF production in the vocal control circuit. BDNF produced in the HVc could mediate effects of estrogens on the differentiation of HVc cells by para- and autocrine actions. In adult canaries the volume of HVc was shown to be affected by BDNF (23). Furthermore, BDNF produced in the HVC could affect vocal control areas connected to the HVc after retro- and anterograde transport.
Because in most of the RA-projecting neurons in the HVc trkB mRNA is not expressed, BDNF is unlikely to act as an autocrine signal in these cells. In contrast, RA cells are potential targets of anterogradely acting BDNF because BDNF mRNA is expressed in RA-projecting neurons of the HVc and because RA cells express trkB mRNA (data presented here) and are responsive to exogenous BDNF (28). The previous finding of BDNF-immunopositive somata in the lMAN, the anterograde transport of exogenous BDNF from the lMAN to the RA, and the rescue of RA neurons by exogenous BDNF after lesion of the lMAN prompted Johnson et al. (28) to assume that BDNF is provided by the lMAN to RA cells. However, we found that RA-projecting neurons of the lMAN do not express BDNF mRNA, and, in a recent study, BDNF immunostaining of lMAN cells could not be confirmed in young males (36). Furthermore, neither target cells of the RA nor RA cells exhibited a substantial expression level of BDNF mRNA.
The estrogen-dependent BDNF mRNA expression only in the HVc points to the HVc as the pacemaker for the estrogen-dependent fraction of the development of the forebrain vocal system. Anterogradely acting BDNF provided by RA-projecting neurons of the HVc could explain how estrogens affect the growth of RA neurons during the period of RA innervation by HVc neurons (6, 7), although the intracellular estrogen receptors are not expressed by RA cells (8–12). Consistent with our suggestion, the estrogen-induced increase of the RA volume is partially impaired after HVc lesion in juvenile females (37), and local intracerebral implants of estrogen in or near the HVc also masculinize the size of RA neurons (38). By triggering the BDNF expression in the HVc of male zebra finches, estrogens could be involved in the development of the male song system.
The scenario of the HVc as a predominant and estrogen-dependent source of BDNF for the song control system and the transport of BDNF in the vocal control circuit also could explain the increase of the BDNF protein level in area X and lMAN after P30 (36) although BDNF mRNA is not expressed or only is expressed at a very low level in these song control areas. The developmental increase of BDNF immunoreactivity in the lMAN of juvenile males (36) could be due to the retrograde transport of BDNF that was delivered from HVc neurons into the RA. Consistent with this assumption, we found that RA-projecting neurons of the lMAN express trkB mRNA. The source of BDNF immunoreactivity around unlabeled area X cell bodies in juvenile males (36) could be area X-projecting neurons of the HVc, some of which we found to express BDNF mRNA (F.D., Y.F., R.M., and M.G., unpublished results).
Other estrogen-dependent factors than BDNF that could be involved in the differentiation of cells within the song control nuclei might include paracrine-acting agents, which are produced by estrogen receptor-containing cells located caudal of HVc and dorsal of RA. Such an estrogen-dependent mechanism is expected to work in the subventricular zone of the adult canary forebrain, affecting new migrating neurons (39). However, further experiments must show to what extent such humoral factors can diffuse in the zebra finch forebrain. The limited time window and area specificity of the estrogen-inducible BDNF expression in the song control system now gives us a handle to work out molecular mechanisms that are responsible for the estrogen-induced differentiation of the vocal control system in the male and female zebra finch.
Acknowledgments
We acknowledge the excellent technical assistance of Mrs. S. Aschenbrenner and C. Voigt and thank Dr. T. Weber for producing computer-assisted overlays. We thank Drs. M. Konishi, R. A. Hughes, and E. Akutagawa for critically reading the manuscript. Further, we thank Dr. R. Janse for his suggestions concerning the quantifications of the autoradiographic results.
ABBREVIATIONS
- AR
androgen receptor
- BDNF
brain-derived neurotrophic factor
- HVc
high vocal center
- KS
Kolmogorow–Smirnow two-sample test
- lMAN
lateral portion of the magnocellular nucleus of the anterior neostriatum
- RA
nucleus robustus of the archistriatum
- P
posthatching day
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Konishi M. Neuron. 1989;3:541–549. doi: 10.1016/0896-6273(89)90264-x. [DOI] [PubMed] [Google Scholar]
- 2.Bottjer S W, Arnold A P. Annu Rev Neurosci. 1997;20:459–481. doi: 10.1146/annurev.neuro.20.1.459. [DOI] [PubMed] [Google Scholar]
- 3.Sossinka R, Pröve E, Kalberlah H H. Z Tierpsychol. 1975;39:259–264. [PubMed] [Google Scholar]
- 4.Korsia S, Bottjer S W. J Neurosci. 1991;11:2362–2371. doi: 10.1523/JNEUROSCI.11-08-02362.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottjer S W, Hewer S J. J Neurobiol. 1992;23:337–353. doi: 10.1002/neu.480230402. [DOI] [PubMed] [Google Scholar]
- 6.Adkins-Regan E, Mansukhani V, Seiwert C, Thompson R. J Neurobiol. 1994;25:865–877. doi: 10.1002/neu.480250710. [DOI] [PubMed] [Google Scholar]
- 7.Balthazart J, Absil P, Fiasse V, Ball G F. Behavior. 1994;131:225–260. [Google Scholar]
- 8.Burkhardt-Holm P, Kafitz K W, Güttinger H R, Schachner M. NeuroReport. 1995;6:433–436. doi: 10.1097/00001756-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Merten M D P, Stocker-Buschina S. Brain Res. 1995;671:317–320. doi: 10.1016/0006-8993(94)01370-w. [DOI] [PubMed] [Google Scholar]
- 10.Arnold A P, Wade J, Grisham W, Jacobs E C, Campagnoni A T. Dev Neurosci. 1996;18:124–136. doi: 10.1159/000111400. [DOI] [PubMed] [Google Scholar]
- 11.Gahr M, Flügge G, Güttinger H-R. Brain Res. 1987;402:173–177. doi: 10.1016/0006-8993(87)91063-8. [DOI] [PubMed] [Google Scholar]
- 12.Nordeen K W, Nordeen E J, Arnold A P. J Neurobiol. 1987;18:569–582. doi: 10.1002/neu.480180607. [DOI] [PubMed] [Google Scholar]
- 13.Gahr M, Konishi M. Proc Natl Acad Sci USA. 1988;85:7380–7383. doi: 10.1073/pnas.85.19.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gahr M. NeuroReport. 1996;7:2469–2473. doi: 10.1097/00001756-199611040-00013. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs E C, Arnold A P, Campagnoni A T. J Steroid Biochem Mol Biol. 1996;59:135–145. doi: 10.1016/s0960-0760(96)00096-9. [DOI] [PubMed] [Google Scholar]
- 16.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 17.Bonhoeffer T. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- 18.Lewin G R, Barde Y A. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 19.Marty S, da Penha Berzaghi M, Berninger B. Trends Neurosci. 1997;20:198–202. doi: 10.1016/s0166-2236(96)01026-0. [DOI] [PubMed] [Google Scholar]
- 20.Heymach J V, Jr, Barres B A. Nature (London) 1997;389:789–791. doi: 10.1038/39743. [DOI] [PubMed] [Google Scholar]
- 21.Toran-Allerand C D. Dev Neurosci. 1996;18:36–48. doi: 10.1159/000111393. [DOI] [PubMed] [Google Scholar]
- 22.Sohrabji F, Miranda R C G, Toran-Allerand C D. Proc Natl Acad Sci USA. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasika S, Alvarez-Buylla A, Nottebohm F. Neuron. 1999;22:53–82. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 24.Gurney M E. J Neurosci. 1981;1:658–673. doi: 10.1523/JNEUROSCI.01-06-00658.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wade J, Schlinger B A, Hodges L, Arnold A P. Gen Comp Endocrinol. 1994;94:53–61. doi: 10.1006/gcen.1994.1059. [DOI] [PubMed] [Google Scholar]
- 26.Gahr M, Metzdorf R. Brain Res Bull. 1997;44:509–517. doi: 10.1016/s0361-9230(97)00233-5. [DOI] [PubMed] [Google Scholar]
- 27.Metzdorf R, Gahr M, Fusani L. J Comp Neurol. 1999;407:115–129. [PubMed] [Google Scholar]
- 28.Johnson F, Hohmann S E, DiStefano P S, Bottjer S W. J Neurosci. 1997;17:2101–2111. doi: 10.1523/JNEUROSCI.17-06-02101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baxter G T, Radeke M J, Kuo R C, Makrides V, Hinkle B, Hoang R, Medina-Selby A, Coit D, Valenzuela P, Feinstein S C. J Neurosci. 1997;15:2683–2690. doi: 10.1523/JNEUROSCI.17-08-02683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold A P. J Histochem Cytochem. 1981;29:207–211. doi: 10.1177/29.1A_SUPPL.7288157. [DOI] [PubMed] [Google Scholar]
- 31.Coggeshall L M. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- 32.Gahr M. Zoology. 1997/98;100:260–272. [Google Scholar]
- 33.Wild J M. Eur J Morphol. 1997;35:303–325. doi: 10.1076/ejom.35.4.303.13077. [DOI] [PubMed] [Google Scholar]
- 34.Pröve E. In: Hormones and Behavior in Higher Vertebrates. Althazart J, Pröve E, Gilles R, editors. Berlin: Springer; 1983. pp. 368–374. [Google Scholar]
- 35.Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger M A. Gen Comp Endocr. 1990;78:93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- 36.Akutagawa E, Konishi M. Proc Natl Acad Sci USA. 1998;95:11429–11434. doi: 10.1073/pnas.95.19.11429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herrmann K, Arnold A P. J Neurobiol. 1991;22:29–39. doi: 10.1002/neu.480220104. [DOI] [PubMed] [Google Scholar]
- 38.Grisham W, Mathews G A, Arnold A P. J Neurobiol. 1994;25:185–196. doi: 10.1002/neu.480250209. [DOI] [PubMed] [Google Scholar]
- 39.Jiang J, McMurty J, Niedzwiecki D, Goldman S A. J Neurobiol. 1998;36:1–15. doi: 10.1002/(sici)1097-4695(199807)36:1<1::aid-neu1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]