Abstract
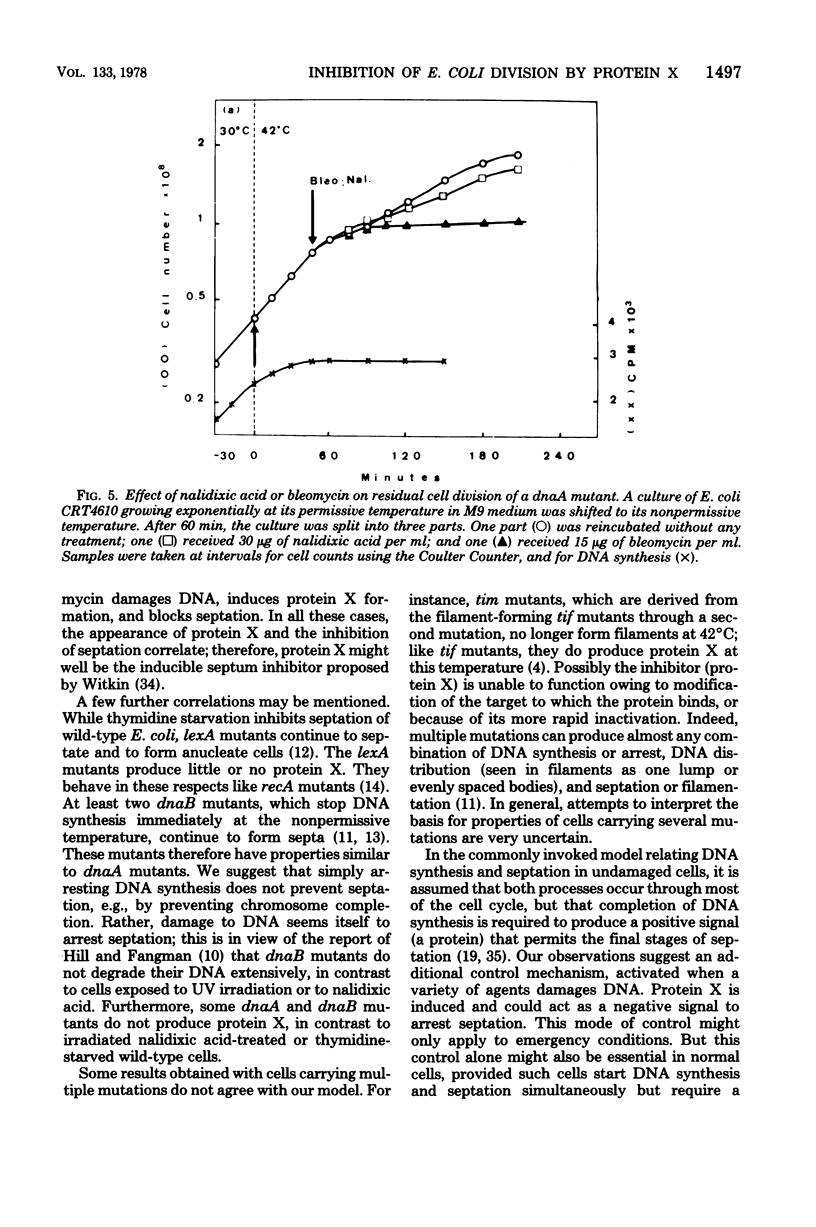
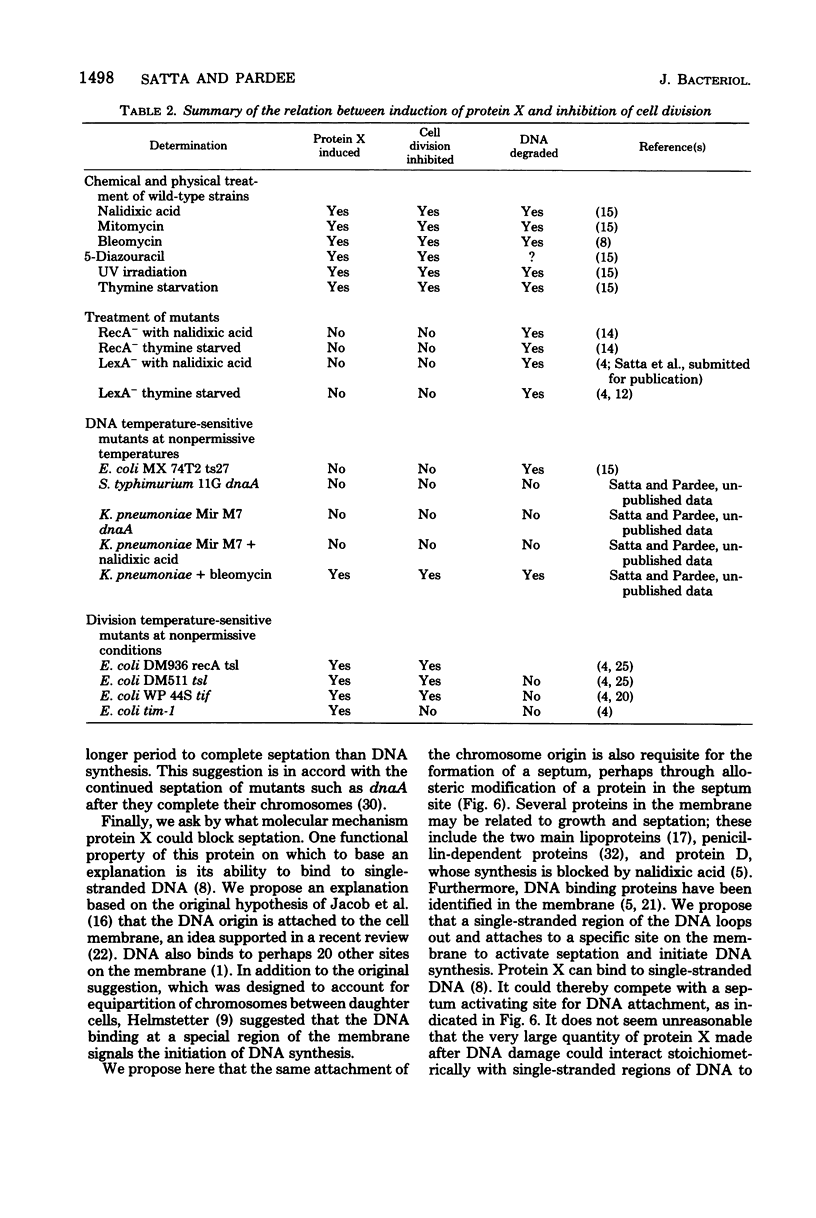
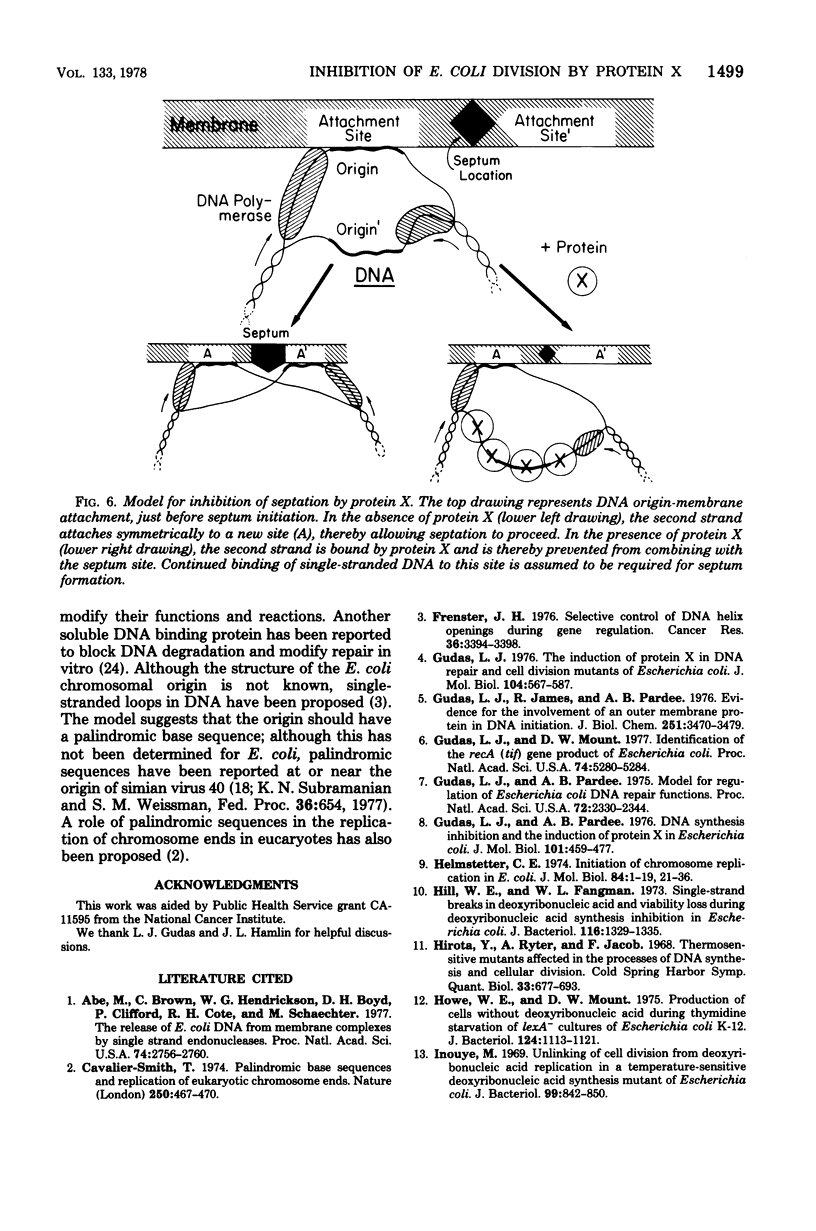
We propose that protein X provides the connection between damage to Escherichia coli DNA and inhibition of septation and cell division. This connection is needed to guarantee that each new bacterium receives a complete DNA copy. We present several new experiments here which demonstrate that the degree to which septation is inhibited following damage to DNA is correlated with the amount of protein X that is produced. Rifampin selectively blocks protein X production. This drug was shown to allow cells whose DNA had been damaged by nalidixic acid to resume septation. Several mutants formed septa-less filaments and also produced protein X at 42°C; rifampin both inhibited their production of protein X and permitted them to form septa and divide. Essentially complementary results were obtained with a dnaA mutant which at 42°C stopped making DNA, did not produce protein X, and continued to divide; added bleomycin degraded DNA, induced protein X, and inhibited septation. These results, as well as previous observations, are all consistent with the proposal that protein X is produced as a consequence of DNA damage and is an inhibitor of septation. We suggest that septation could require binding of a single-stranded region of DNA to a septum site in the membrane. Protein X could block this binding by combining with the DNA. This control could provide an emergency mechanism in addition to the usually proposed coordination in which completion of DNA synthesis creates a positive effector for a terminal step of septation. Or it could be the sole coordinating mechanism, even under unperturbed growth conditions.
Full text
PDF

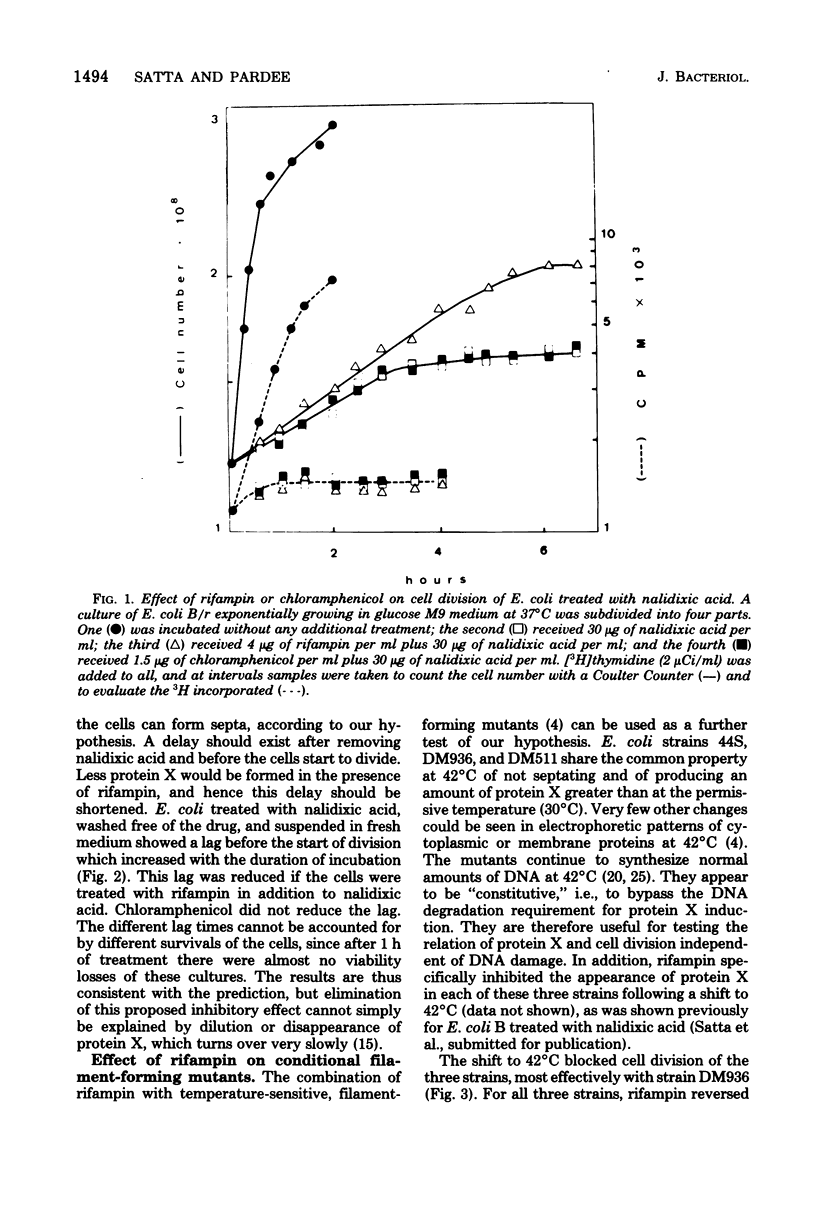
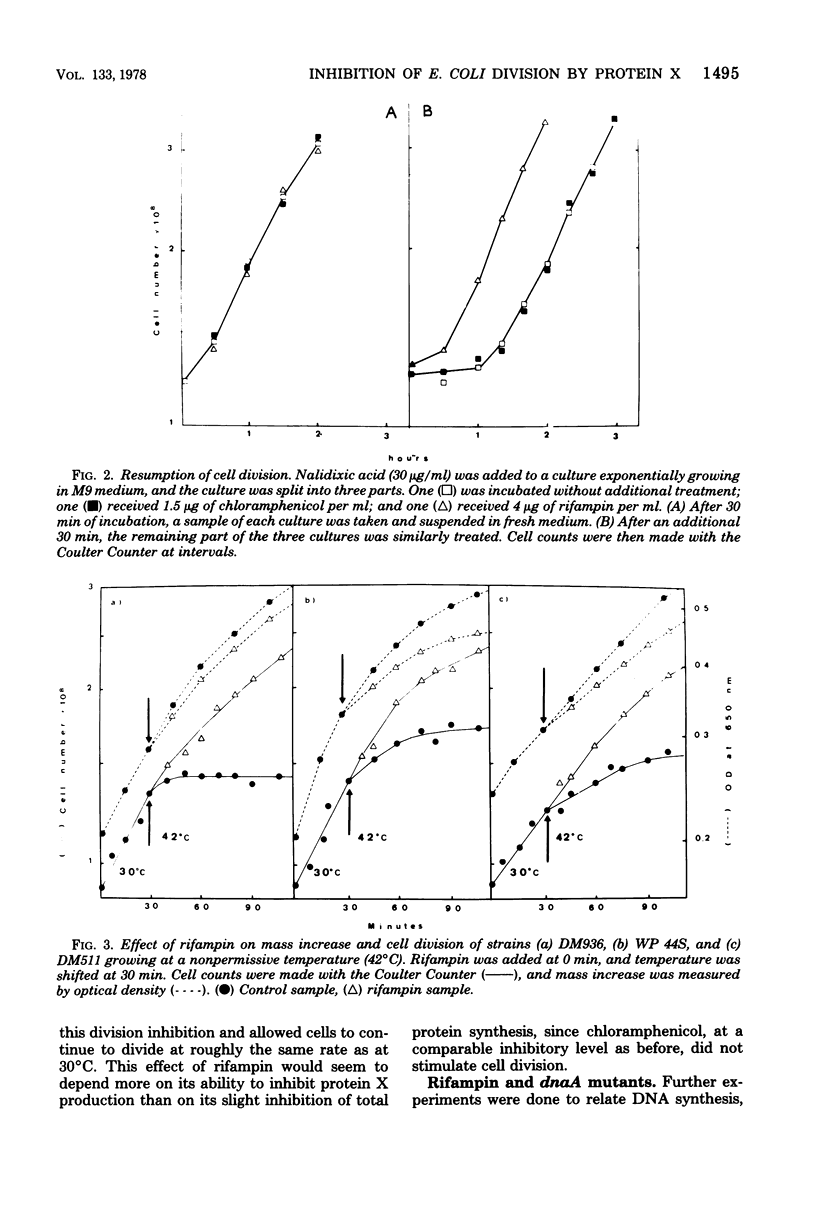
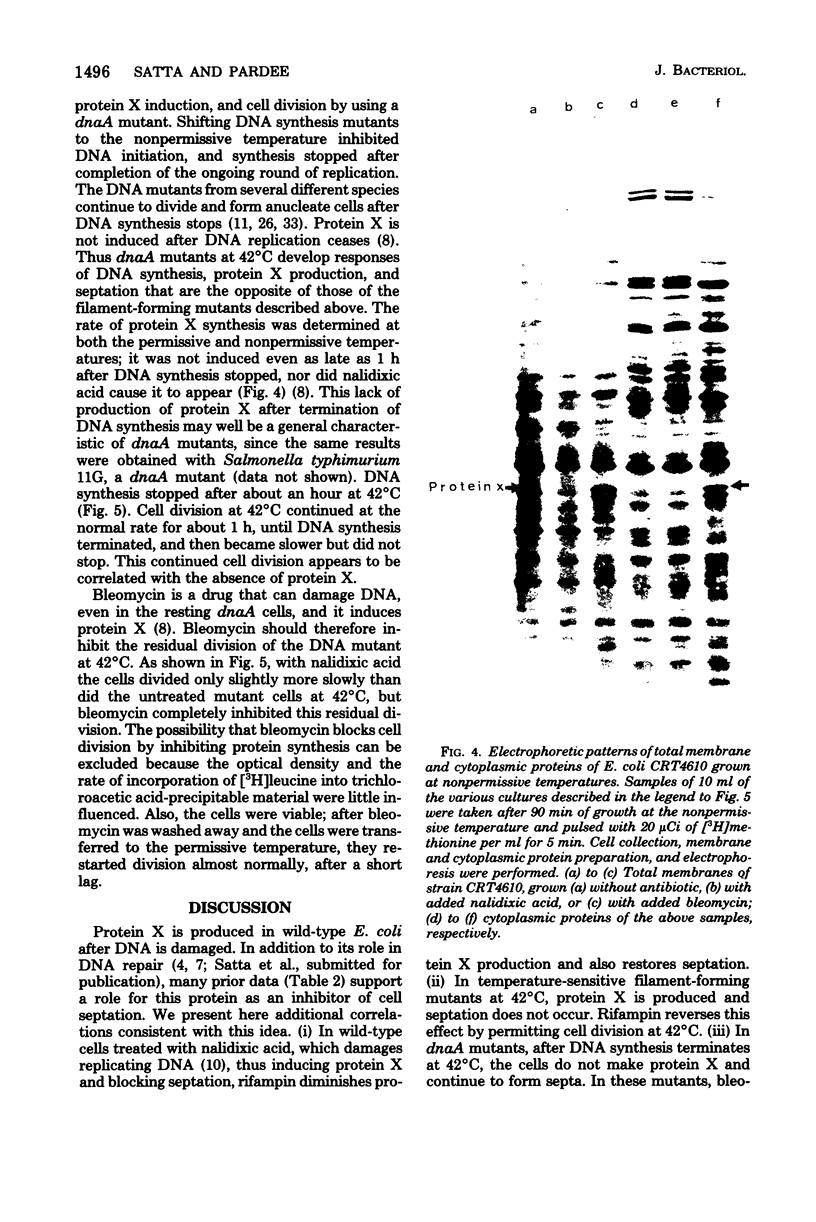

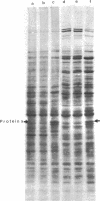
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Brown C., Hendrickson W. G., Boyd D. H., Clifford P., Cote R. H., Schaechter M. Release of Escherichia coli DNA from membrane complexes by single-strand endonucleases. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2756–2760. doi: 10.1073/pnas.74.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T. Palindromic base sequences and replication of eukaryote chromosome ends. Nature. 1974 Aug 9;250(5466):467–470. doi: 10.1038/250467a0. [DOI] [PubMed] [Google Scholar]
- Frenster J. H. Selective control of DNA helix openings during gene regulation. Cancer Res. 1976 Sep;36(9 Pt 2):3394–3398. [PubMed] [Google Scholar]
- Gudas L. J., James R., Paradee A. B. Evidence of the involvement of an outer membrane protein in DNA initiation. J Biol Chem. 1976 Jun 10;251(11):3470–3479. [PubMed] [Google Scholar]
- Gudas L. J., Mount D. W. Identification of the recA (tif) gene product of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5280–5284. doi: 10.1073/pnas.74.12.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Model for regulation of Escherichia coli DNA repair functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2330–2334. doi: 10.1073/pnas.72.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J. The induction of protein X in DNA repair and cell division mutants of Escherichia coli. J Mol Biol. 1976 Jul 5;104(3):567–587. doi: 10.1016/0022-2836(76)90121-2. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. Initiation of chromosome replication in Escherichia coli. II. Analysis of the control mechanism. J Mol Biol. 1974 Mar 25;84(1):21–36. doi: 10.1016/0022-2836(74)90210-1. [DOI] [PubMed] [Google Scholar]
- Hill W. E., Fangman W. L. Single-strand breaks in deoxyribonucleic acid and viability loss during deoxyribonucleic acid synthesis inhibition in Escherichia coli. J Bacteriol. 1973 Dec;116(3):1329–1335. doi: 10.1128/jb.116.3.1329-1335.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Howe W. E., Mount D. W. Production of cells without deoxyribonucleic acid during thymidine starvation of lexA- cultures of Escherichia coli K-12. J Bacteriol. 1975 Dec;124(3):1113–1121. doi: 10.1128/jb.124.3.1113-1121.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Pardee A. B. Changes of membrane proteins and their relation to deoxyribonucleic acid synthesis and cell division of Escherichia coli. J Biol Chem. 1970 Nov 10;245(21):5813–5819. [PubMed] [Google Scholar]
- Inouye M. Pleiotropic effect of the rec A gene of Escherichia coli: uncoupling of cell division from deoxyribonucleic acid replication. J Bacteriol. 1971 May;106(2):539–542. doi: 10.1128/jb.106.2.539-542.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. Unlinking of cell division from deoxyribonucleic acid replication in a temperature-sensitive deoxyribonucleic acid synthesis mutant of Escherichia coli. J Bacteriol. 1969 Sep;99(3):842–850. doi: 10.1128/jb.99.3.842-850.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R. Identification of an outer membrane protein of Escherichia coli, with a role in the coordination of deoxyribonucleic acid replication and cell elongation. J Bacteriol. 1975 Nov;124(2):918–929. doi: 10.1128/jb.124.2.918-929.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Roychoudhury R., Wu R. Nucleotide sequence with elements of an unusual two-fold rotational symmetry in the region of origin of replication of SV40 DNA+. Biochem Biophys Res Commun. 1976 Apr 5;69(3):678–686. doi: 10.1016/0006-291x(76)90929-3. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Donachie W. D. Chromosome replication, transcription and control of cell division in Escherichia coli. Nat New Biol. 1973 May 23;243(125):100–103. [PubMed] [Google Scholar]
- Kirby E. P., Jacob F., Goldthwait D. A. Prophage induction and filament formation in a mutant strain of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1903–1910. doi: 10.1073/pnas.58.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohiyama M., Kollek R., Goebel W., Kepes A. Escherichia coli membrane proteins with an affinity for deoxyribonucleic acid. J Bacteriol. 1977 Feb;129(2):658–667. doi: 10.1128/jb.129.2.658-667.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz P. J., Schaechter M. The attachment of the bacterial chromosome to the cell membrane. Int Rev Cytol. 1975;41:1–28. doi: 10.1016/s0074-7696(08)60964-x. [DOI] [PubMed] [Google Scholar]
- Mackay V., Linn S. Selective inhibition of the dnase activity of the recBC enzyme by the DNA binding protein from Escherichia coli. J Biol Chem. 1976 Jun 25;251(12):3716–3719. [PubMed] [Google Scholar]
- McEntee K., Hesse J. E., Epstein W. Identification and radiochemical purification of the recA protein of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3979–3983. doi: 10.1073/pnas.73.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mound D. W., Walker A. C., Kosel C. Suppression of lex mutations affecting deoxyribonucleic acid repair in Escherichia coli K-12 by closely linked thermosensitive mutations. J Bacteriol. 1973 Nov;116(2):950–956. doi: 10.1128/jb.116.2.950-956.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Anucleate cell production and surface extension in a temperature-sensitive chromosome initiation mutant of Bacillus subtilis. J Bacteriol. 1975 Sep;123(3):1218–1234. doi: 10.1128/jb.123.3.1218-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Canepari P., Fontana R., Calegari L. Envelope protein alterations in a conditional mutant of Klebsiella pneumoniae with pH dependent morphology and temperature dependent division. Ann Microbiol (Paris) 1974 Sep;125 B(2):259–273. [PubMed] [Google Scholar]
- Sedgwick S. G. Ultraviolet inducible protein associated with error prone repair in E. coli B. Nature. 1975 May 22;255(5506):349–350. doi: 10.1038/255349a0. [DOI] [PubMed] [Google Scholar]
- Shannon K. P., Rowbury R. J. Alteration of the rate of cell division independent of the rate of DNA synthesis in a mutant of Salmonella typhimurium. Mol Gen Genet. 1972;115(2):122–125. doi: 10.1007/BF00277291. [DOI] [PubMed] [Google Scholar]
- Siccardi A. G., Shapiro B. M. On the process of cellular division in Escherichia coli. IV. Altered protein composition and turnover of the membranes of thermosensitive mutants defective in chromosomal replication. J Mol Biol. 1971 Mar 28;56(3):475–490. doi: 10.1016/0022-2836(71)90395-0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Rowbury R. J. A mutant in the initiation of DNA synthesis in Salmonella typhimurium. J Gen Microbiol. 1970 Dec;64(2):127–138. doi: 10.1099/00221287-64-2-127. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. The radiation sensitivity of Escherichia coli B: a hypothesis relating filament formation and prophage induction. Proc Natl Acad Sci U S A. 1967 May;57(5):1275–1279. doi: 10.1073/pnas.57.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Pritchard R. H. Changes in cell size and shape associated with changes in the replication time of the chromosome of Escherichia coli. J Bacteriol. 1973 May;114(2):824–837. doi: 10.1128/jb.114.2.824-837.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]