Abstract
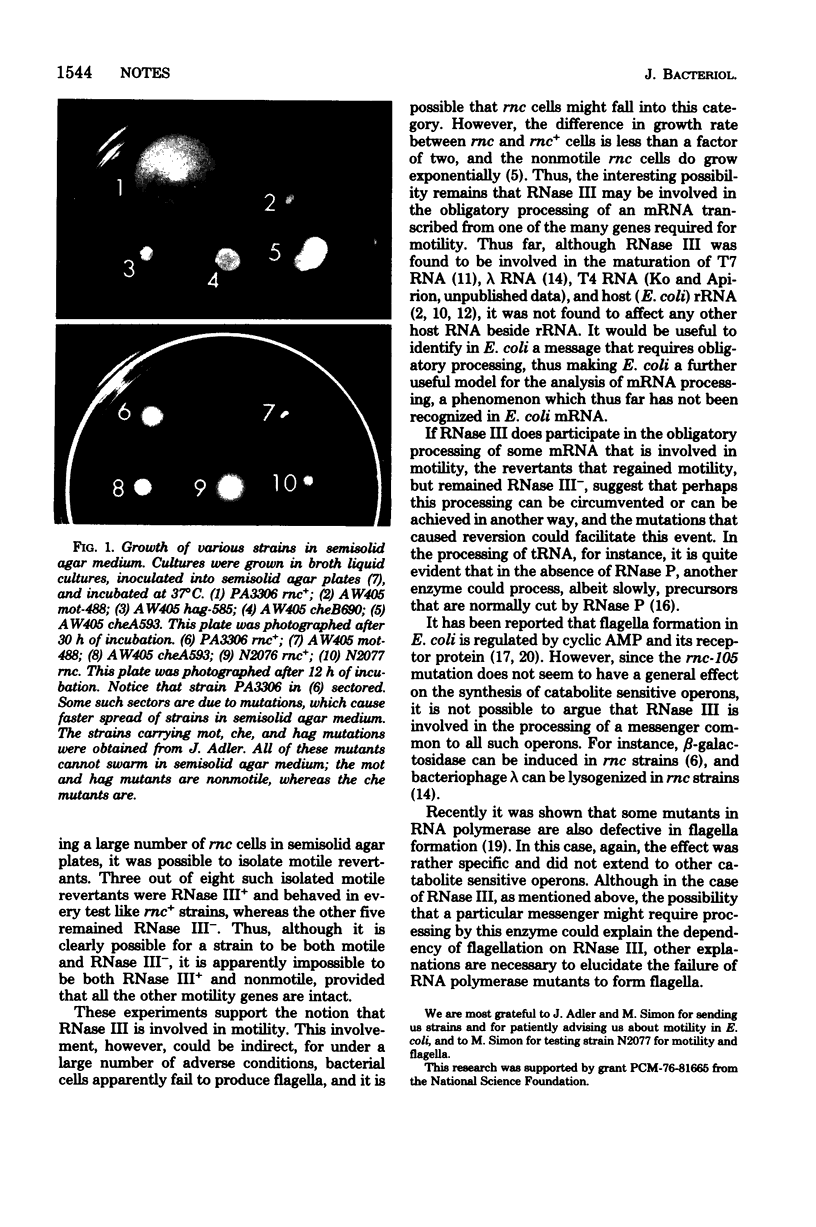
Mutants of Escherichia coli deficient in ribonuclease III are nonmotile. All transductants and revertants that regained ribonuclease III also regained motility, and all transductants that remained or became rnc are nonmotile, although only some of the revertants that regained motility also became ribonuclease III+.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Apirion D., Neil J., Watson N. Revertants from RNase III negative strains of Escherichia coli. Mol Gen Genet. 1976 Dec 8;149(2):201–210. doi: 10.1007/BF00332890. [DOI] [PubMed] [Google Scholar]
- Apirion D. The fate of mRNA and rRNA in Escherichia coli. Brookhaven Symp Biol. 1975 Jul;(26):286–306. [PubMed] [Google Scholar]
- Apirion D., Watson N. Analysis of an Escherichia coli strain carrying physiologically compensating mutations one of which causes an altered ribonuclease 3. Mol Gen Genet. 1974;132(2):89–104. doi: 10.1007/BF00272175. [DOI] [PubMed] [Google Scholar]
- Apirion D., Watson N. Mapping and characterization of a mutation in Escherichia coli that reduces the level of ribonuclease III specific for double-stranded ribonucleic acid. J Bacteriol. 1975 Oct;124(1):317–324. doi: 10.1128/jb.124.1.317-324.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apirion D., Watson N. Unaltered stability of newly synthesized RNA in strains of Escherichia coli missing a ribonuclease specific for double-stranded RNA. Mol Gen Genet. 1975;136(4):317–326. doi: 10.1007/BF00341716. [DOI] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J., Dahl M. M. Nonchemotactic mutants of Escherichia coli. J Bacteriol. 1967 Jan;93(1):390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C. Chemotaxis in bacteria. Annu Rev Biophys Bioeng. 1975;4(00):119–136. doi: 10.1146/annurev.bb.04.060175.001003. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Processing transcription, and translation of bacteriophage T7 messenger RNAs. Brookhaven Symp Biol. 1975 Jul;(26):267–276. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegenheimer P., Watson N., Apirion D. Multiple pathways for primary processing of ribosomal RNA in Escherichia coli. J Biol Chem. 1977 May 10;252(9):3064–3073. [PubMed] [Google Scholar]
- Komeda Y., Silverman M., Simon M. Genetic analysis of Escherichia coli K-12 region I flagellar mutants. J Bacteriol. 1977 Sep;131(3):801–808. doi: 10.1128/jb.131.3.801-808.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozeron H. A., Anevski P. J., Apirion D. Antitermination and absence of processing of the leftward transcript of coliphage lambda in the RNAase III-deficient host. J Mol Biol. 1977 Jan 15;109(2):359–365. doi: 10.1016/s0022-2836(77)80039-9. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S. Genetics of chemotactic behavior in bacteria. Cell. 1975 Mar;4(3):183–188. doi: 10.1016/0092-8674(75)90166-x. [DOI] [PubMed] [Google Scholar]
- Schedl P., Primakoff P., Roberts J. Processing of E. coli tRNA precursors. Brookhaven Symp Biol. 1975 Jul;(26):53–76. [PubMed] [Google Scholar]
- Silverman M., Simon M. Characterization of Escherichia coli flagellar mutants that are insensitive to catabolite repression. J Bacteriol. 1974 Dec;120(3):1196–1203. doi: 10.1128/jb.120.3.1196-1203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Genetic mapping of a mutation that causes ribonucleases III deficiency in Escherichia coli. J Bacteriol. 1975 Oct;124(1):307–316. doi: 10.1128/jb.124.1.307-316.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Ito K., Yura T., Suzuki T., Iino T. Ribonucleic acid polymerase mutant of Escherichia coli defective in flagella formation. J Bacteriol. 1977 Oct;132(1):254–261. doi: 10.1128/jb.132.1.254-261.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Gots J. S. Requirement of adenosine 3', 5'-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970 Aug;103(2):513–516. doi: 10.1128/jb.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]