Abstract
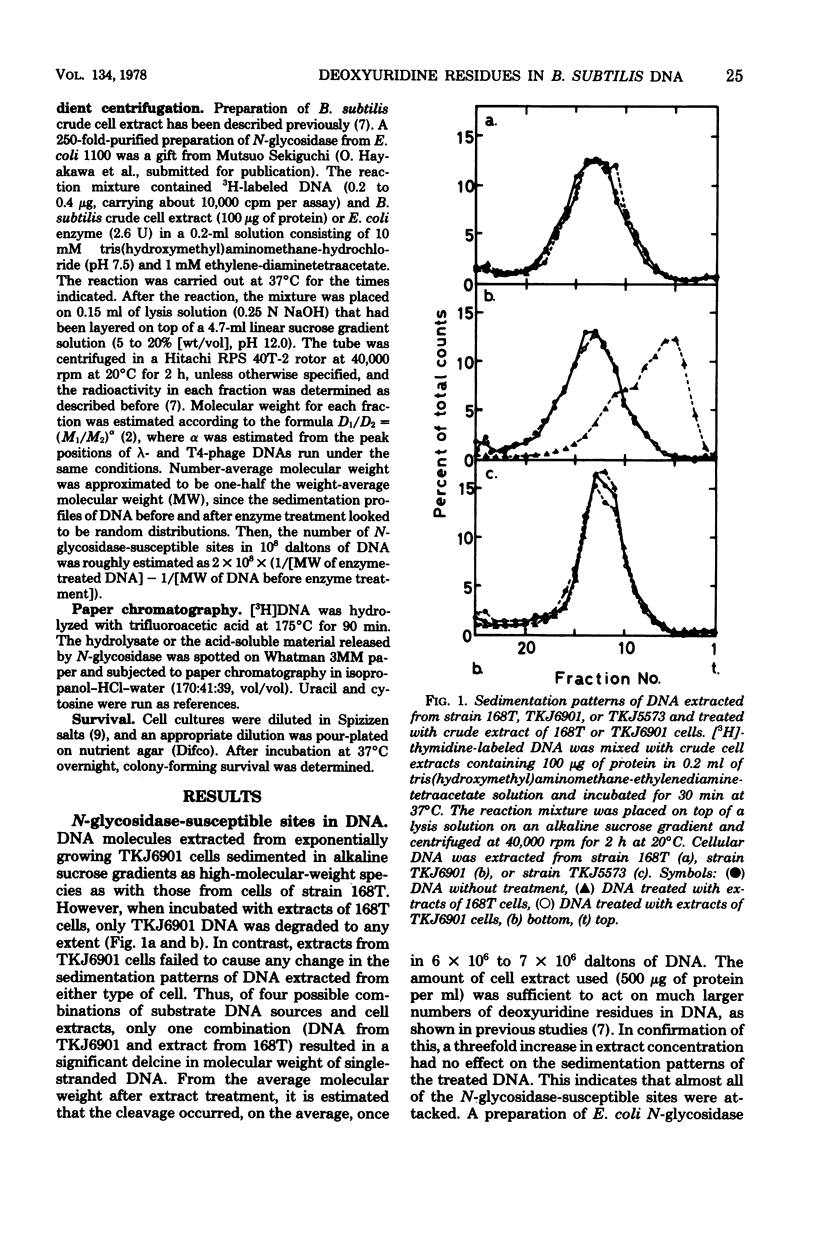
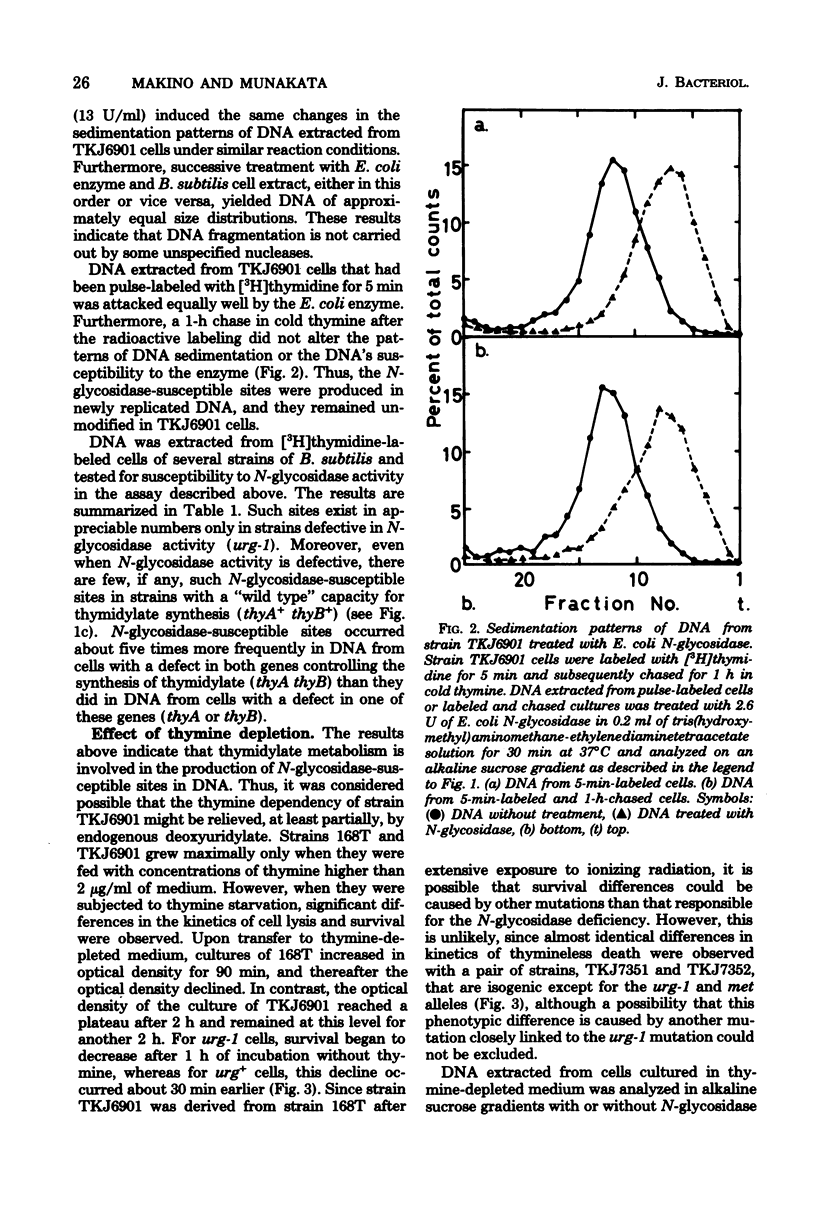
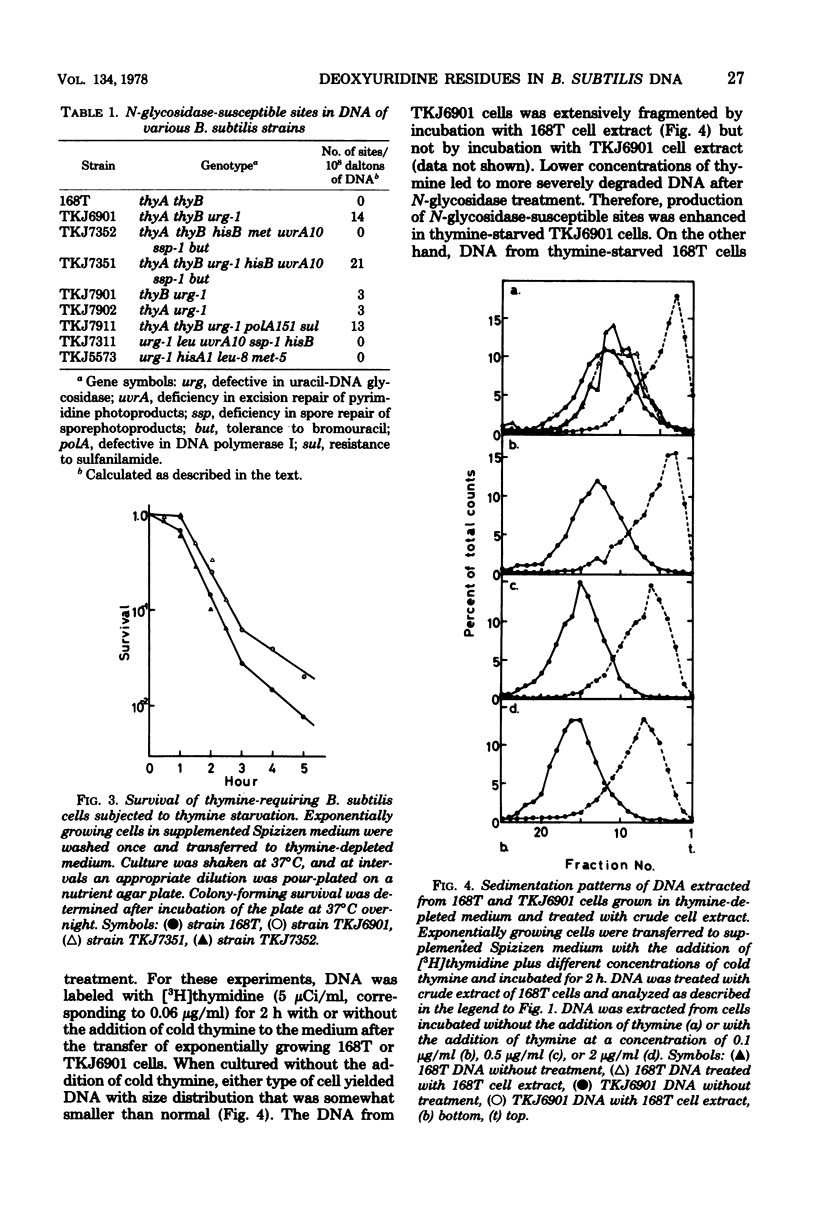
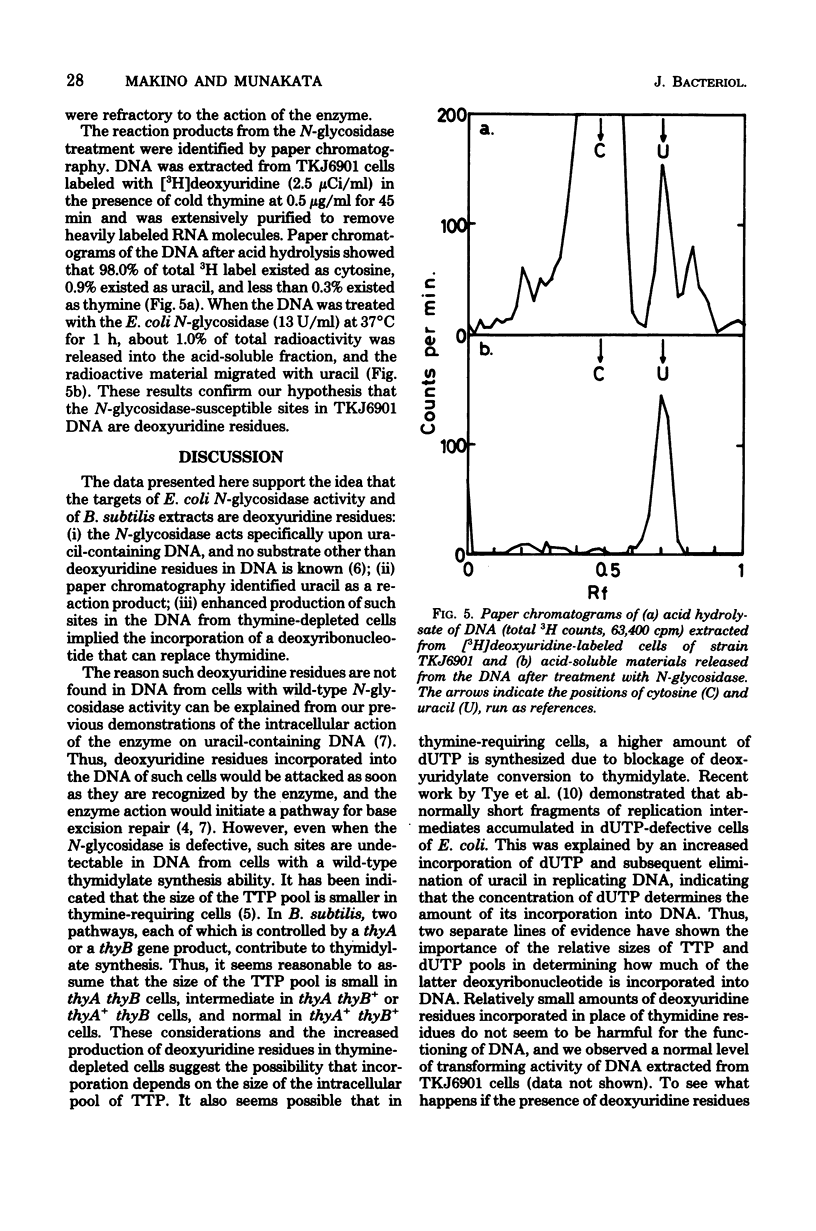
DNA extracted from exponentially growing cells of thymine-requiring Bacillus subtilis strains with defective N-glycosidase activity for deoxyuridine residues in DNA was subjected to the action of N-glycosidase in vitro and analyzed by sedimentation in alkaline sucrose gradients. The sites attacked by N-glycosidase occurred once per 6 X 10(6) to 7 X 10(6) daltons of DNA from cells cultured in the presence of growth-supporting concentrations of thymine. The number of N-glycosidase-susceptible sites increased when the thymine concentration in the medium was lowered. Parallel to this observation, the N-glycosidase-defective mutant cells were less apt to show the detrimental effect due to thymine depletion than were the parental cells. Such sites were not detected in DNA from cells with a normal N-glycosidase activity or with a "wild type" capacity for thymidylate synthesis. The results are interpreted to mean that cells defective for thymidylate synthesis incorporate dUTP in place of TTP in DNA and that the deoxyuridine residues, once incorporated, remain in the DNA in the absence of N-glycosidase activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier F., Sicard N. Interference of dna ts mutations of Escherichia coli with thymineless death. J Bacteriol. 1975 Dec;124(3):1198–1204. doi: 10.1128/jb.124.3.1198-1204.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A. T., Wiener D., Werner R. Synthesis of small polynucleotide chains in thymine-depleted bacteria. J Mol Biol. 1975 Jun 15;95(1):45–61. doi: 10.1016/0022-2836(75)90334-4. [DOI] [PubMed] [Google Scholar]
- Duncan J., Hamilton L., Friedberg E. C. Enzymatic degradation of uracil-containing DNA. II. Evidence for N-glycosidase and nuclease activities in unfractionated extracts of Bacillus subtilis. J Virol. 1976 Aug;19(2):338–345. doi: 10.1128/jvi.19.2.338-345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrati-Elizur E., Borenstein S. Velocity of chromosome replication in thymine-requiring and independent strains of Bacillus subtilis. J Bacteriol. 1971 Apr;106(1):58–64. doi: 10.1128/jb.106.1.58-64.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isolation and characterization of a Bacillus subtilis mutant with a defective N-glycosidase activity for uracil-containing deoxyribonucleic acid. J Bacteriol. 1977 Aug;131(2):438–445. doi: 10.1128/jb.131.2.438-445.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Ljungquist S., Siegert W., Nyberg B., Sperens B. DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem. 1977 May 25;252(10):3286–3294. [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye B. K., Nyman P. O., Lehman I. R., Hochhauser S., Weiss B. Transient accumulation of Okazaki fragments as a result of uracil incorporation into nascent DNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):154–157. doi: 10.1073/pnas.74.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. C., Farmer J. L., Rothman F. Thymidylate synthesis and aminopterin resistance in Bacillus subtilis. J Bacteriol. 1966 Jul;92(1):186–196. doi: 10.1128/jb.92.1.186-196.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



