Abstract
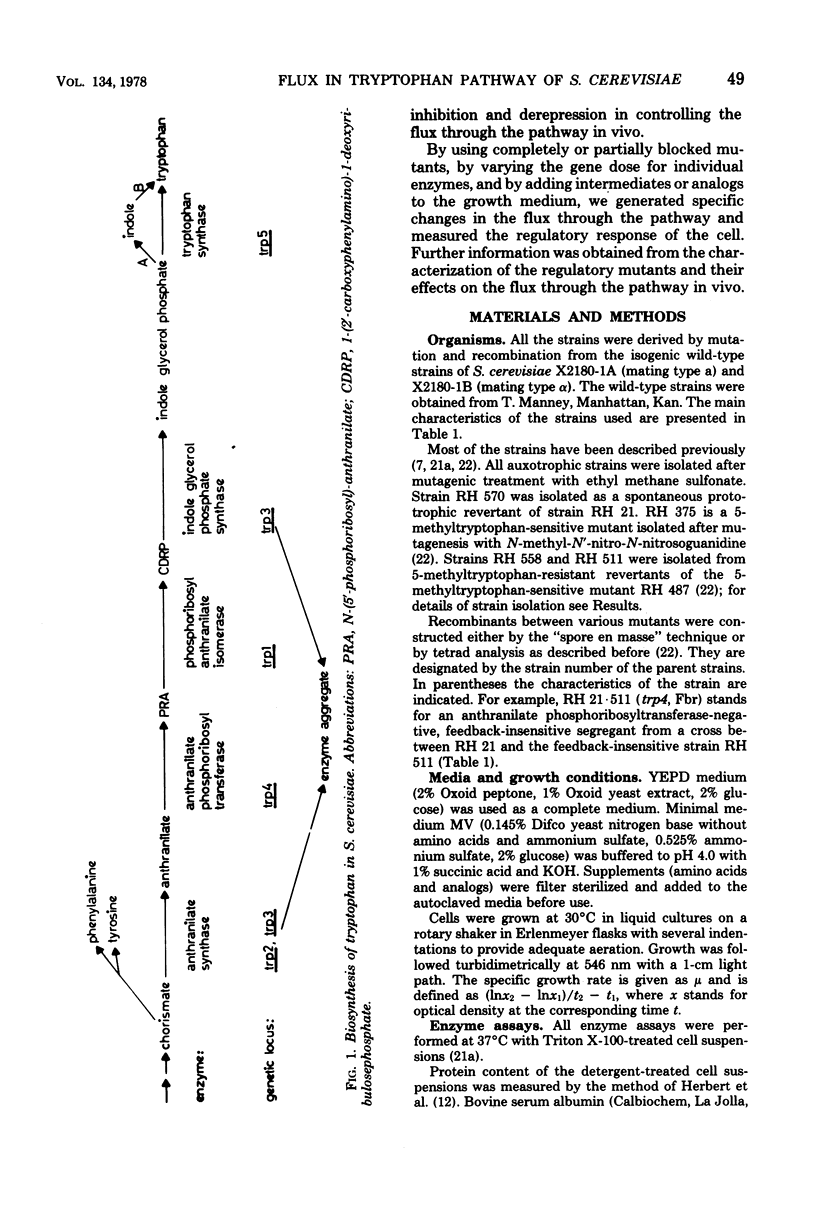
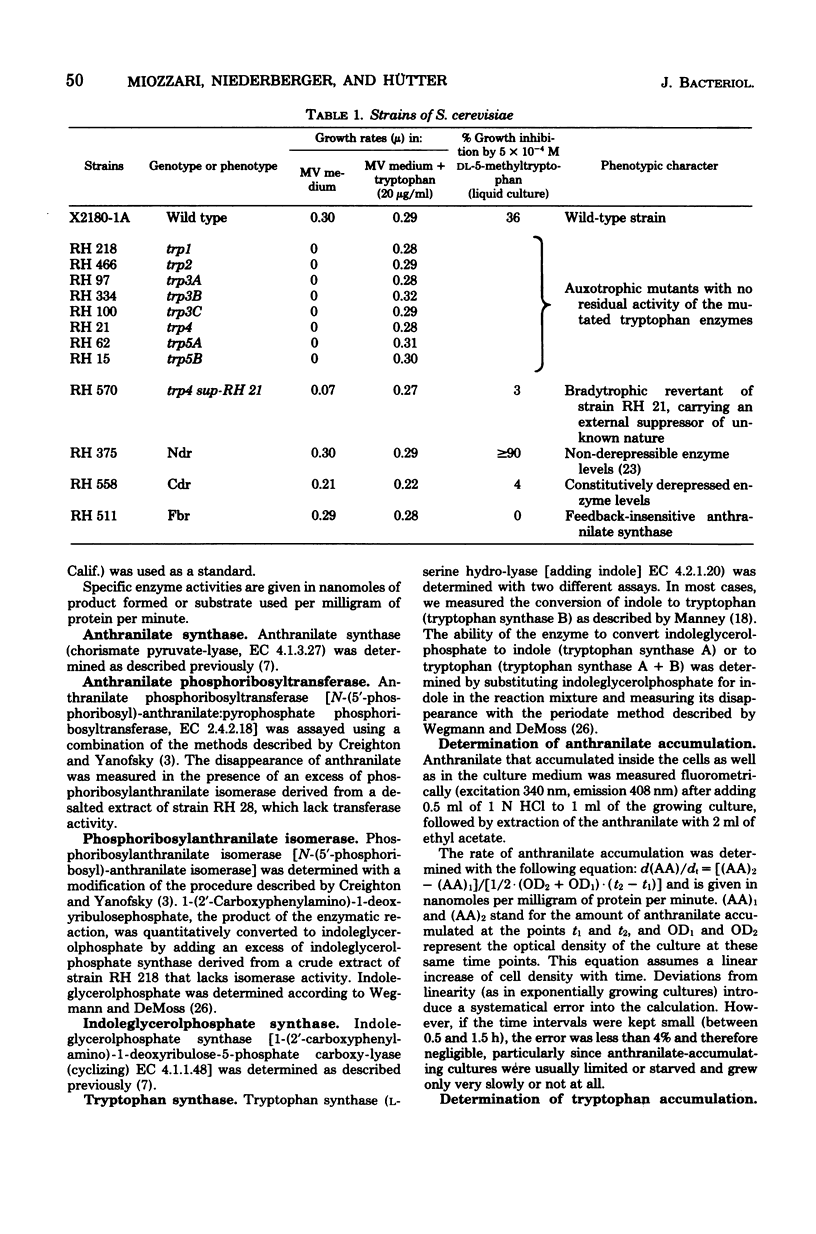
Enzyme derepression and feedback inhibition of the first enzyme are the regulatory mechanisms demonstrated for the tryptophan pathway in Saccharomyces cerevisiae. The relative contributions of the two mechanisms to the control of the flux through the pathway in vivo were analyzed by (i) measuring feedback inhibition of anthranilate synthase in vivo, (ii) determining the effect of regulatory mutations on the level of the tryptophan pool and the flux through the pathway, and (iii) varying the gene dose of individual enzymes of the pathway at the tetraploid level. We conclude that the flux through the pathway is adjusted to the rate of protein synthesis by means of feedback inhibition of the first enzyme by the end product, tryptophan. The synthesis of the tryptophan enzymes could not be repressed below a basal level by tryptophan supplementation of the media. The enzymes are present in excess. Increasing or lowering the concentration of individual enzymes had no noticeable influencing on the overall flux to tryptophan. The uninhibited capacity of the pathway could be observed both upon relieving feedback inhibition by tryptophan limitation and in feedback-insensitive mutants. It exceeded the rate of consumption of the amino acid on minimal medium by a factor of three. Tryptophan limitation caused derepression of four of the five tryptophan enzymes and, as a consequence, led to a further increase in the capacity of the pathway. However, because of the large reserve capacity of the "repressed" pathway, tryptophan limitation could not be imposed on wild-type cells without resorting to the use of analogs. Our results, therefore, suggest that derepression does not serve as an instrument for the specific regulation of the flux through the tryptophan pathway.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carsiotis M., Jones R. F. Cross-pathway regulation: tryptophan-mediated control of histidine and arginine biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1974 Sep;119(3):889–892. doi: 10.1128/jb.119.3.889-892.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delforge J., Messenguy F., Wiame J. M. The regulation of arginine biosynthesis in Saccharomyces cerevisiae. The specificity of argR- mutations and the general control of amino-acid biosynthesis. Eur J Biochem. 1975 Sep 1;57(1):231–239. doi: 10.1111/j.1432-1033.1975.tb02295.x. [DOI] [PubMed] [Google Scholar]
- Doy C. H., Cooper J. M. Aromatic biosynthesis in yeast. I. The synthesis of tryptophan and the regulation of this pathway. Biochim Biophys Acta. 1966 Oct 31;127(2):302–316. [PubMed] [Google Scholar]
- Duntze W., Manney T. R. Two mechanisms of allelic complementation among tryptophan synthetase mutants of Saccharomyces cerevisiae. J Bacteriol. 1968 Dec;96(6):2085–2093. doi: 10.1128/jb.96.6.2085-2093.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantes P. A., Roberts L. M., Huetter R. Free tryptophan pool and tryptophan biosynthetic enzymes in Saccharomyces cerevisiae. Arch Microbiol. 1976 Mar 19;107(2):207–214. doi: 10.1007/BF00446842. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C. Concentrations of intermediary metabolites in yeast. Biochimie. 1973;55(2):205–211. doi: 10.1016/s0300-9084(73)80393-1. [DOI] [PubMed] [Google Scholar]
- Heilmann H. D., Lingens F. Reinigung und Eigenschaften der 3-Hydroxy-anthranilat-Oxygenase aus Saccharomyces cerevisiae. Hoppe Seylers Z Physiol Chem. 1968 Feb;349(2):223–230. [PubMed] [Google Scholar]
- Heilmann H. D., Lingens F. Zur Regulation der Nicotinsäure-Biosynthese in Saccharomyces cerevisiae. Hoppe Seylers Z Physiol Chem. 1968 Feb;349(2):231–236. [PubMed] [Google Scholar]
- Lester G. Regulation of tryptophan biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1971 Jul;107(1):193–202. doi: 10.1128/jb.107.1.193-202.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingens F., Goebel W., Uesseler H. Regulation der Biosynthese der aromatischen Aminosäuren in Saccharomyces cerevisiae. 2. Repression, Induktion und Aktivierung. Eur J Biochem. 1967 May;1(3):363–374. doi: 10.1111/j.1432-1033.1967.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Manney T. R., Duntze W., Janosko N., Salazar J. Genetic and biochemical studies of partially active tryptophan synthetase mutants of Saccharomyces cerevisiae. J Bacteriol. 1969 Aug;99(2):590–596. doi: 10.1128/jb.99.2.590-596.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manney T. R. Regulation of factors that influence the in vitro stability of tryptophan synthetase from yeast. J Bacteriol. 1968 Aug;96(2):403–408. doi: 10.1128/jb.96.2.403-408.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchett W. H. Inhibition of tryptophan synthetase by indoleacrylic acid. J Bacteriol. 1972 Apr;110(1):146–154. doi: 10.1128/jb.110.1.146-154.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchett W. H., Turner J. R., Wiley W. R. The role of tryptophan in the physiology of Neurospora. Yale J Biol Med. 1968 Feb;40(4):257–283. [PMC free article] [PubMed] [Google Scholar]
- Miozzari G., Niederberger P., Hütter R. Action of tryptophan analogues in Saccharomyces cerevisiae. Arch Microbiol. 1977 Dec 15;115(3):307–316. doi: 10.1007/BF00446457. [DOI] [PubMed] [Google Scholar]
- Schürch-Rathgeb Y. Der trp3-Locus von Saccharomyces cerevisiae. Arch Genet (Zur) 1972;45(3):129–159. [PubMed] [Google Scholar]
- Schürch A., Miozzari J., Hütter R. Regulation of tryptophan biosynthesis in Saccharomyces cerevisiae: mode of action of 5-methyl-tryptophan and 5-methyl-tryptophan-sensitive mutants. J Bacteriol. 1974 Mar;117(3):1131–1140. doi: 10.1128/jb.117.3.1131-1140.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. R., Matchett W. H. Alteration of tryptophan-mediated regulation in Neurospora crassa by indoleglycerol phosphate. J Bacteriol. 1968 May;95(5):1608–1614. doi: 10.1128/jb.95.5.1608-1614.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegman J., DeMoss J. A. The enzymatic conversion of anthranilate to indolylglycerol phosphate in Neurospora crassa. J Biol Chem. 1965 Oct;240(10):3781–3788. [PubMed] [Google Scholar]
- Weiss R. L., Kukora J. R., Adams J. The relationship between enzyme activity, cell geometry, and fitness in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1975 Mar;72(3):794–798. doi: 10.1073/pnas.72.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfner M., Yep D., Messenguy F., Fink G. R. Integration of amino acid biosynthesis into the cell cycle of Saccharomyces cerevisiae. J Mol Biol. 1975 Aug 5;96(2):273–290. doi: 10.1016/0022-2836(75)90348-4. [DOI] [PubMed] [Google Scholar]



