Abstract
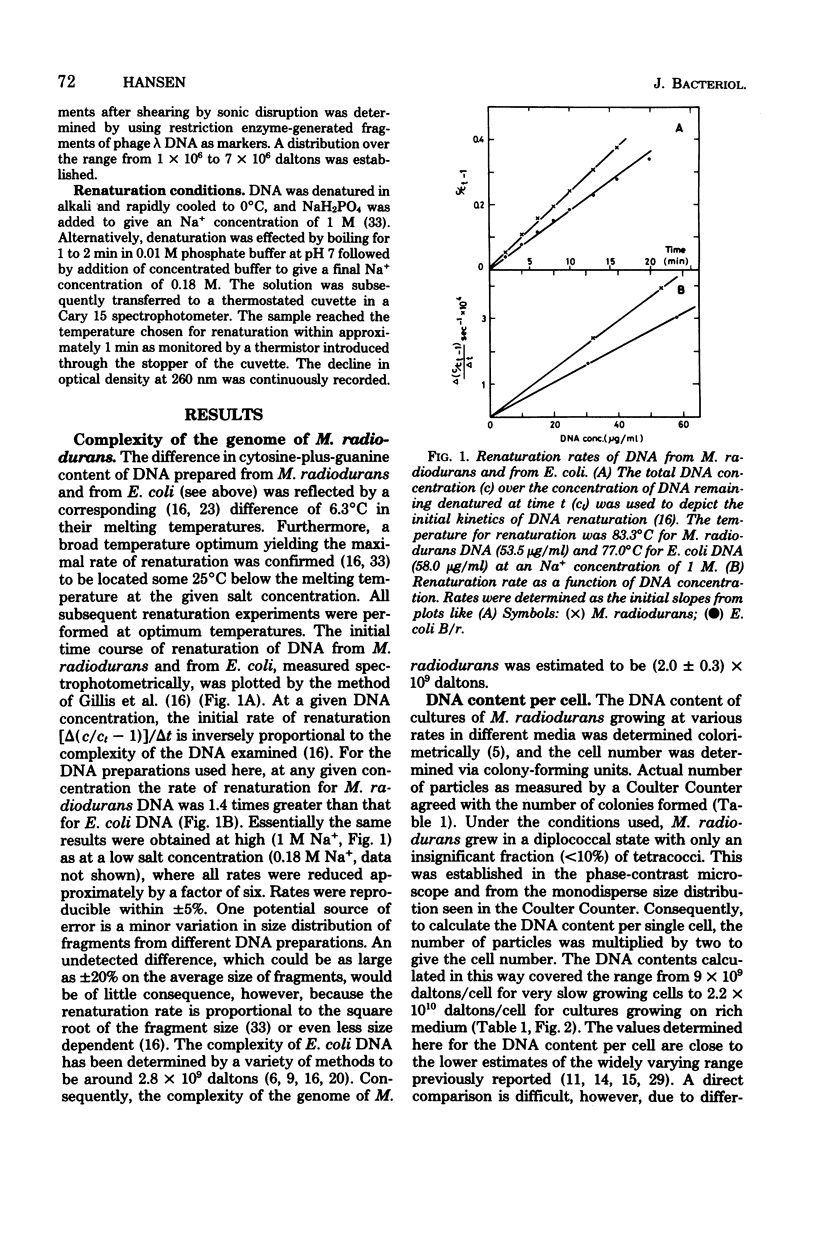
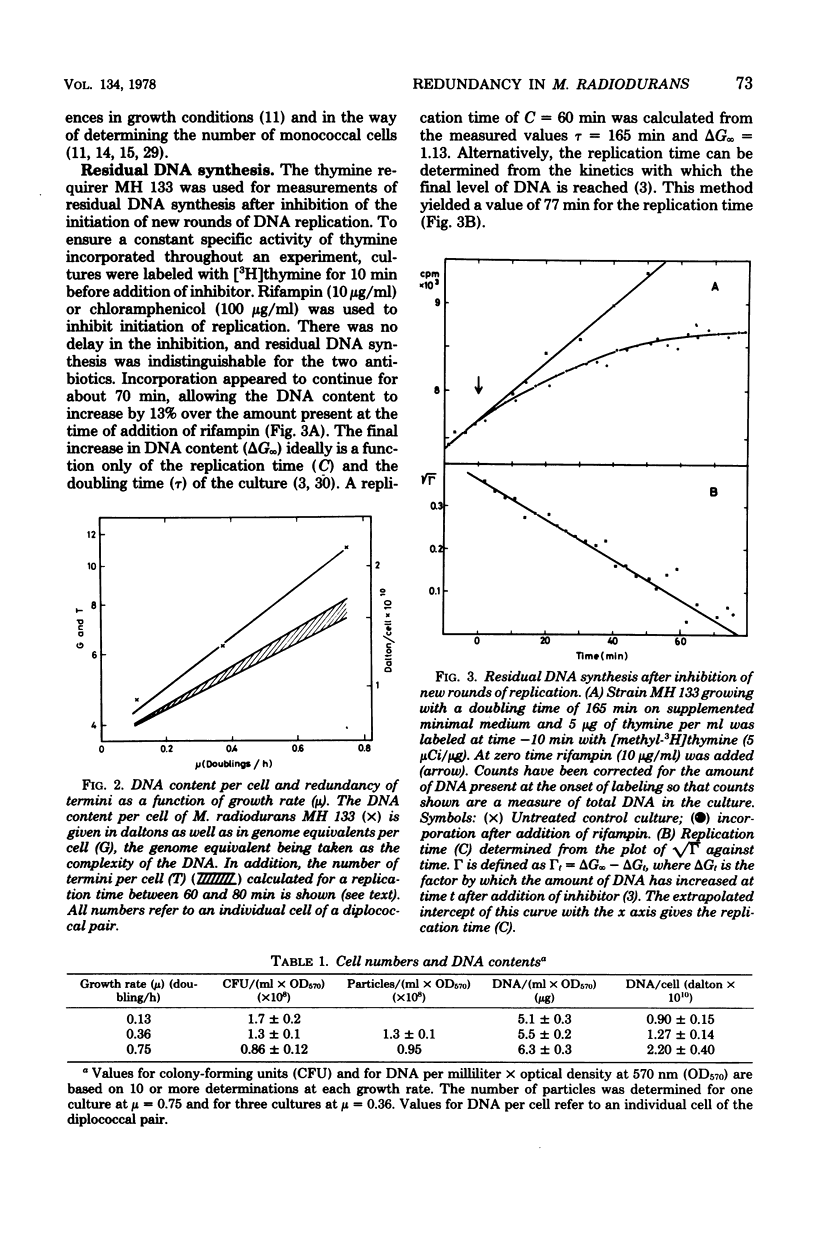
The complexity of the genome of Micrococcus radiodurans was determined to be (2.0 +/- 0.3) X 10(9) daltons by DNA renaturation kinetics. The number of genome equivalents of DNA per cell was calculated from the complexity and the content of DNA. A lower limit of four genome equivalents per cell was approached with decreasing growth rate. Thus, no haploid stage appeared to be realized in this organism. The replication time was estimated from the kinetics and amount of residual DNA synthesis after inhibiting initiation of new rounds of replication. From this, the redundancy of terminal genetic markers was calculated to vary with growth rate from four to approximately eight copies per cell. All genetic material, including the least abundant, is thus multiply represented in each cell. The potential significance of the maintenance in each cell of multiple gene copies is discussed in relation to the extreme radiation resistance of M. radiodurans.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K. The resistance of Micrococcus radiodurans to ultraviolet radiation. 3. A repair mechanism. Biochim Biophys Acta. 1966 Jul 20;123(1):26–33. doi: 10.1016/0005-2787(66)90155-9. [DOI] [PubMed] [Google Scholar]
- Bremer H., Churchward G. Deoxyribonucleic acid synthesis after inhibition of initiation of rounds of replication in Escherichia coli B/r. J Bacteriol. 1977 May;130(2):692–697. doi: 10.1128/jb.130.2.692-697.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell A. D., Feldschreiber P., Dean C. J. DNA-membrane association and the repair of double breaks in x-irradiated Micrococcus radiodurans. Biochim Biophys Acta. 1971 Sep 30;247(1):38–53. doi: 10.1016/0005-2787(71)90805-7. [DOI] [PubMed] [Google Scholar]
- Chandler M., Bird R. E., Caro L. The replication time of the Escherichia coli K12 chromosome as a function of cell doubling time. J Mol Biol. 1975 May 5;94(1):127–132. doi: 10.1016/0022-2836(75)90410-6. [DOI] [PubMed] [Google Scholar]
- Churchward G., Bremer H. Determination of deoxyribonucleic acid replication time in exponentially growing Escherichia coli B/r. J Bacteriol. 1977 Jun;130(3):1206–1213. doi: 10.1128/jb.130.3.1206-1213.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Driedger A. A. The DNA content of single cells of Micrococcus radiodurans. Can J Microbiol. 1970 Nov;16(11):1136–1137. doi: 10.1139/m70-192. [DOI] [PubMed] [Google Scholar]
- Driedger A. A. The ordered growth pattern of microcolonies of Micrococcus radiodurans: first generation sectoring of induced lethal mutations. Can J Microbiol. 1970 Nov;16(11):1133–1135. doi: 10.1139/m70-191. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Bruce A. K. Deoxyribonucleic acid-protein ratio and radioresistance in Micrococcus radiodurans. J Bacteriol. 1972 Mar;109(3):1310–1312. doi: 10.1128/jb.109.3.1310-1312.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M. L., Bruce A. K. The relationship of radioresistance to balanced growth-rate in Micrococcus radiodurans. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19(2):111–121. doi: 10.1080/09553007114550161. [DOI] [PubMed] [Google Scholar]
- Gillis M., De Ley J., De Cleene M. The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem. 1970 Jan;12(1):143–153. doi: 10.1111/j.1432-1033.1970.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Hansen M. T., Bennett P. M., von Meyenburg K. Intracistronic polarity during dissociation of translation from transcription in Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):589–604. doi: 10.1016/0022-2836(73)90225-8. [DOI] [PubMed] [Google Scholar]
- Kapp D. S., Smith K. C. Chemical nature of chain breaks produced in DNA by x-irradiation in vitro. Radiat Res. 1970 Apr;42(1):34–49. [PubMed] [Google Scholar]
- Kitayama S., Matsuyama A. Possibility of the repair of double-strand scissions in Micrococcus radiodurans DNA caused by gamma-rays. Biochem Biophys Res Commun. 1968 Nov 8;33(3):418–422. doi: 10.1016/0006-291x(68)90588-3. [DOI] [PubMed] [Google Scholar]
- Lett J. T., Caldwell I., Little J. G. Repair of x-ray damage to the DNA in Micrococcus radiodurans: the effect of 5-bromodeoxyuridine. J Mol Biol. 1970 Mar;48(3):395–408. doi: 10.1016/0022-2836(70)90053-7. [DOI] [PubMed] [Google Scholar]
- Little J. G., Hanawalt P. C. Thymineless death and ultraviolet sensitivity in Micrococcus radiodurans. J Bacteriol. 1973 Jan;113(1):233–240. doi: 10.1128/jb.113.1.233-240.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- MOSELEY B. E., LASER H. REPAIR OF X-RAY IN MICROCOCCUS RADIODURANS. Proc R Soc Lond B Biol Sci. 1965 Apr 13;162:210–222. doi: 10.1098/rspb.1965.0035. [DOI] [PubMed] [Google Scholar]
- MOSELEY B. E., SCHEIN A. H. RADIATION RESISTANCE AND DEOXYRIBONUCLEIC ACID BASE COMPOSITION OF MICROCOCCUS RADIODURANS. Nature. 1964 Sep 19;203:1298–1299. doi: 10.1038/2031298a0. [DOI] [PubMed] [Google Scholar]
- Moseley B. E., Copland H. J. The rate of recombination repair and its relationship to the radiation-induced delay in DNA synthesis in Micrococcus radiodurans. J Gen Microbiol. 1976 Apr;93(2):251–258. doi: 10.1099/00221287-93-2-251. [DOI] [PubMed] [Google Scholar]
- Moseley B. E., Mattingly A., Copland H. J. Sensitization to radiation by loss of recombination ability in a temperature-sensitive DNA mutant of Micrococcus radiodurans held at its restrictive temperature. J Gen Microbiol. 1972 Sep;72(2):329–338. doi: 10.1099/00221287-72-2-329. [DOI] [PubMed] [Google Scholar]
- Pritchard R. H., Zaritsky A. Effect of thymine concentration on the replication velocity of DNA in a thymineless mutant of Escherichia coli. Nature. 1970 Apr 11;226(5241):126–131. doi: 10.1038/226126a0. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- Sweet D. M., Moseley B. E. The resistance of Micrococcus radiodurans to killing and mutation by agents which damage DNA. Mutat Res. 1976 Feb;34(2):175–186. doi: 10.1016/0027-5107(76)90122-6. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Zeuthen J., Pato M. L. Replication of the F'lac sex factor in the cell cycle of Escherichia coli. Mol Gen Genet. 1971;111(3):242–255. doi: 10.1007/BF00433109. [DOI] [PubMed] [Google Scholar]



