Abstract
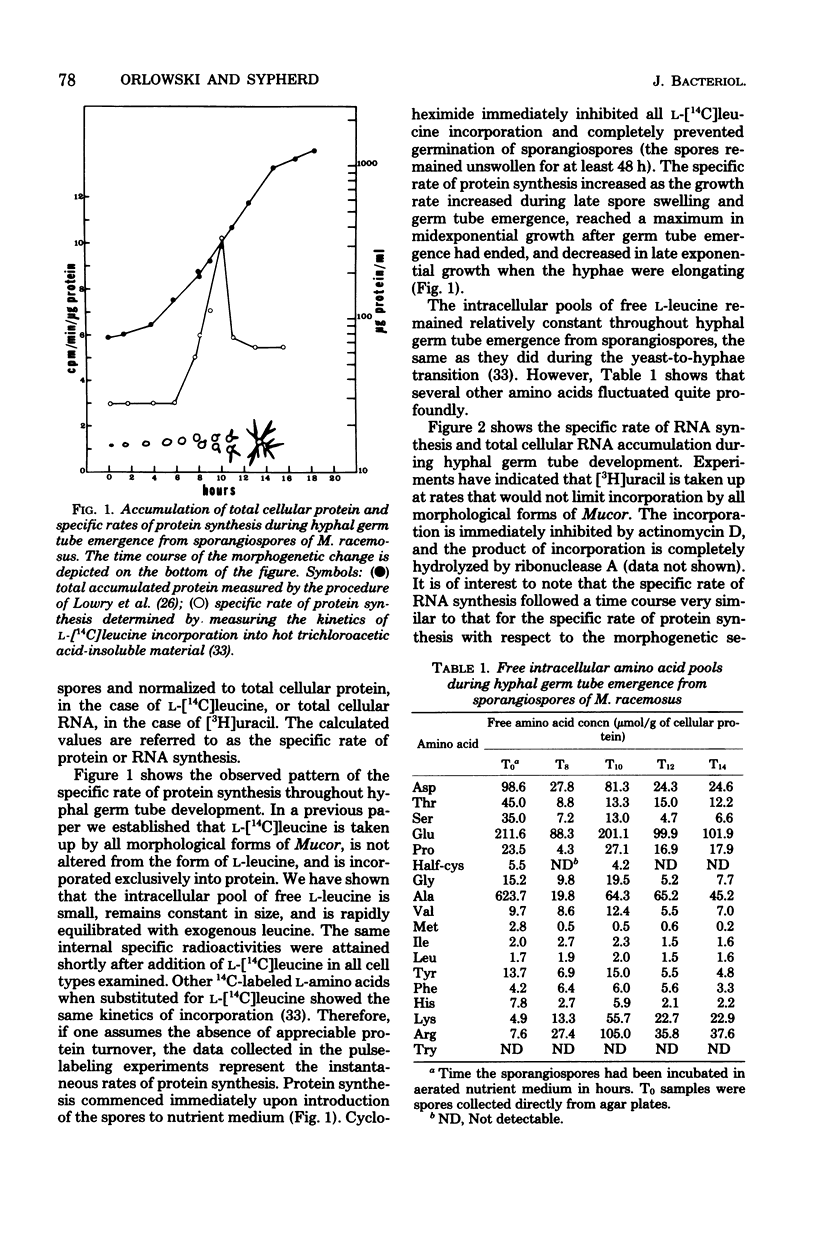
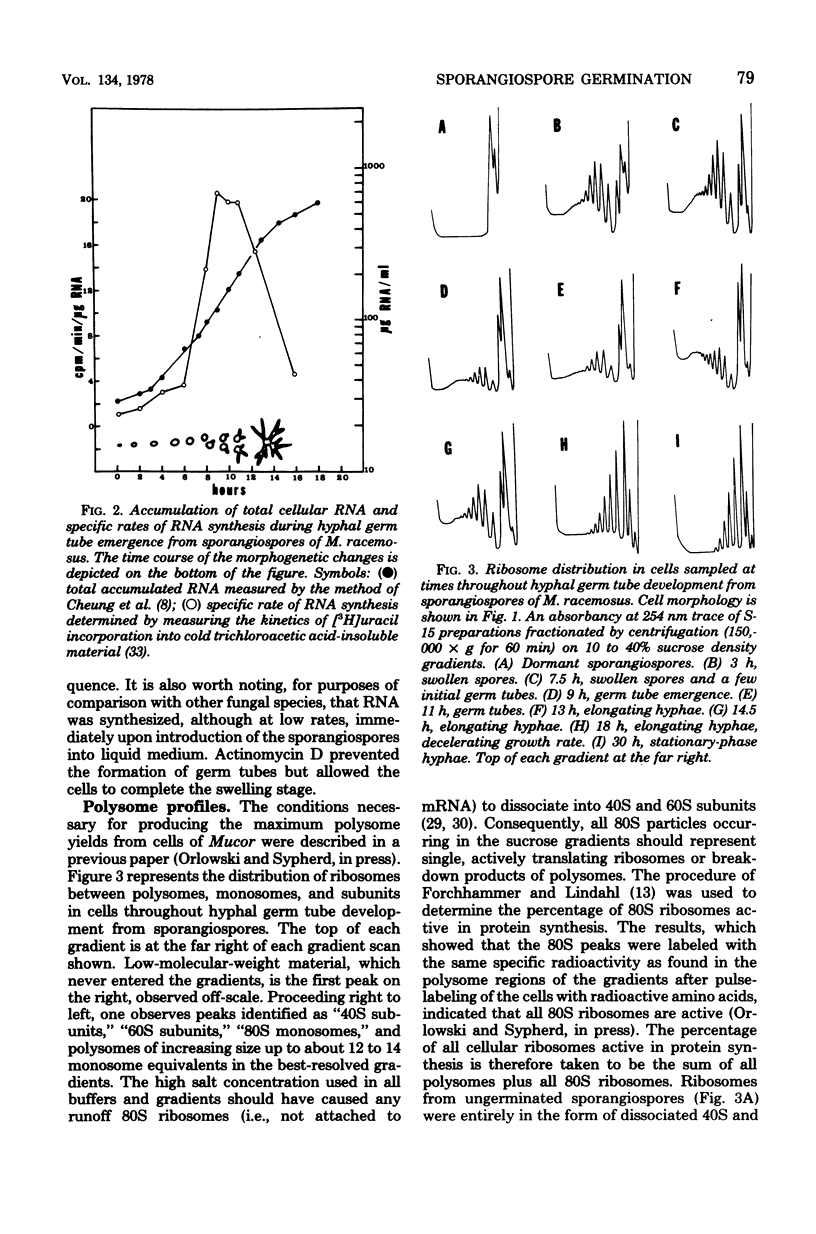
Protein and RNA syntheses were examined during hyphal germ tube emergence from sporangiospores of a dimorphic phycomycete, Mucor racemosus. Both classes of macromolecules were synthesized immediately upon introduction of the dormant sporangiospores into nutrient medium. The specific rates of synthesis of both protein and RNA accelerated during initial germ tube emergence and reached a maximum when the emergence of new germ tubes ended. The specific rates of synthesis later decreased during further hyphal elongation. The distribution of ribosomes between active polysomes and monosomes and inactive subunits was determined by sucrose density gradient centrifugation, and the rate of amino acid addition to nascent polypeptide chains was calculated throughout the developmental sequence. The results showed that both the percentage of ribosomes active in protein synthesis and the velocity of ribosome movement along the mRNA were continuously adjusted throughout hyphal germ tube development. The free intracellular amino acid pools were measured throughout development. Alanine, glutamate, and aspartate were present at very high concentrations in the dormant spores but were rapidly depleted during hyphal germ tube emergence. The results of these studies are discussed in relation to hyphal germ tube development from yeast cells of Mucor and dormant spores of other fungal species.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberghina F. A., Sturani E., Gohlke J. R. Levels and rates of synthesis of ribosomal ribonucleic acid, transfer ribonucleic acid, and protein in Neurospora crassa in different steady states of growth. J Biol Chem. 1975 Jun 25;250(12):4381–4388. [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Induction of yeast-like development in Mucor by carbon dioxide. J Bacteriol. 1962 Oct;84:829–840. doi: 10.1128/jb.84.4.829-840.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon C. W., Sussman A. S. Effects of the self-inhibitor of Dictyostelium discoideum on spore metabolism. J Gen Microbiol. 1973 Jun;76(2):331–344. doi: 10.1099/00221287-76-2-331. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S., Lippman E. Fungal morphogenesis: cell wall construction in Mucor rouxii. Science. 1969 Jul 18;165(3890):302–304. doi: 10.1126/science.165.3890.302. [DOI] [PubMed] [Google Scholar]
- Boehlke K. W., Friesen J. D. Cellular content of ribonucleic acid and protein in Saccharomyces cerevisiae as a function of exponential growth rate: calculation of the apparent peptide chain elongation rate. J Bacteriol. 1975 Feb;121(2):429–433. doi: 10.1128/jb.121.2.429-433.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambl R. M., Van Etten J. L. Protein synthesis during fungal spore germination. V. Evidence that the ungerminated conidiospores of Botryodiplodia theobromae contain messenger ribonucleic acid. Arch Biochem Biophys. 1970 Apr;137(2):442–452. doi: 10.1016/0003-9861(70)90461-3. [DOI] [PubMed] [Google Scholar]
- Cheung S. S., Kobayashi G. S., Schlessinger D., Medoff G. RNA metabolism during morphogenesis in Histoplasma capsulatum. J Gen Microbiol. 1974 Jun;82(2):301–307. doi: 10.1099/00221287-82-2-301. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- Firtel R. A., Baxter L., Lodish H. F. Actinomycin D and the regulation of enzyme biosynthesis during development of Dictyostelium discoideum. J Mol Biol. 1973 Sep 15;79(2):315–327. doi: 10.1016/0022-2836(73)90008-9. [DOI] [PubMed] [Google Scholar]
- Forchhammer J., Lindahl L. Growth rate of polypeptide chains as a function of the cell growth rate in a mutant of Escherichia coli 15. J Mol Biol. 1971 Feb 14;55(3):563–568. doi: 10.1016/0022-2836(71)90337-8. [DOI] [PubMed] [Google Scholar]
- HENNEY H. R., Jr, STORCK R. POLYRIBOSOMES AND MORPHOLOGY IN NEUROSPORA CRASSA. Proc Natl Acad Sci U S A. 1964 Jun;51:1050–1055. doi: 10.1073/pnas.51.6.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschemeyer A. E. Rates of polypeptide chain assembly in liver in vivo: relation to the mechanism of temperature acclimation in Opsanus tau. Proc Natl Acad Sci U S A. 1969 Jan;62(1):128–135. doi: 10.1073/pnas.62.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollomon D. W. Ribonucleic acid synthesis during fungal spore germination. J Gen Microbiol. 1970 Jul;62(1):75–87. doi: 10.1099/00221287-62-1-75. [DOI] [PubMed] [Google Scholar]
- Horikoshi K., Ikeda Y. Studies on the conidia of Aspergillus oryzae. VII. Development of protein synthesizing activity during germination. Biochim Biophys Acta. 1968 Sep 24;166(2):505–511. [PubMed] [Google Scholar]
- Jaworski A. J. Synthesis of polyadenylic acid RNA during zoospore differentiation and germination in Blastocladiella emersonii. Arch Biochem Biophys. 1976 Mar;173(1):201–209. doi: 10.1016/0003-9861(76)90250-2. [DOI] [PubMed] [Google Scholar]
- Knight R. H., Van Etten J. L. Synthesis of ribonucleic acids during the germination of Botryodiplodia theobromae pycnidiospores. J Gen Microbiol. 1976 Aug;96(2):257–267. doi: 10.1099/00221287-95-2-257. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langheinrich W., Ring K. Regulation of amino acid transport in growing cells of Streptomyces hydrogenans. I. Modulation of transport capacity and amino acid pool composition during the growth cycle. Arch Microbiol. 1976 Sep 1;109(3):227–235. doi: 10.1007/BF00446633. [DOI] [PubMed] [Google Scholar]
- Larsen A. D., Sypherd P. S. Cyclic adenosine 3',5'-monophosphate and morphogenesis in Mucor racemosus. J Bacteriol. 1974 Feb;117(2):432–438. doi: 10.1128/jb.117.2.432-438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Lovett J. S. An analysis of protein and RNA synthesis during encystment and outgrowth (germination) of Blastocladiella zoospores. Cell Differ. 1974 Sep;3(3):165–192. doi: 10.1016/0045-6039(74)90028-1. [DOI] [PubMed] [Google Scholar]
- Lingappa B. T., Lingappa Y., Bell E. A self-inhibitor of protein synthesis in the conidia of Glomerella cingulata. Arch Mikrobiol. 1973 Dec 21;94(2):97–107. doi: 10.1007/BF00416685. [DOI] [PubMed] [Google Scholar]
- Lovett J. S. Growth and differentiation of the water mold Blastocladiella emersonii: cytodifferentiation and the role of ribonucleic acid and protein synthesis. Bacteriol Rev. 1975 Dec;39(4):345–404. doi: 10.1128/br.39.4.345-404.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett J. S., Haselby J. A. Molecular weights of the ribosomal ribonucleic acid of fungi. Arch Mikrobiol. 1971;80(3):191–204. doi: 10.1007/BF00410121. [DOI] [PubMed] [Google Scholar]
- Martin S. E., Iandolo J. J. Translational Control of Protein Synthesis in Staphylococcus aureus. J Bacteriol. 1975 Jun;122(3):1136–1143. doi: 10.1128/jb.122.3.1136-1143.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. E. A simple general method to determine the proportion of active ribosomes in eukaryotic cells. Exp Cell Res. 1973 Aug;80(2):496–498. doi: 10.1016/0014-4827(73)90333-9. [DOI] [PubMed] [Google Scholar]
- Martin T. E., Hartwell L. H. Resistance of active yeast ribosomes to dissociation by KCl. J Biol Chem. 1970 Mar 25;245(6):1504–1506. [PubMed] [Google Scholar]
- Mirkes P. E. Polysomes, ribonucleic acid, and protein synthesis during germination of Neurospora crassa conidia. J Bacteriol. 1974 Jan;117(1):196–202. doi: 10.1128/jb.117.1.196-202.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Sypherd P. S. Cyclic guanosine 3',5'-monophosphate in the dimorphic fungus Mucor racemosus. J Bacteriol. 1976 Mar;125(3):1226–1228. doi: 10.1128/jb.125.3.1226-1228.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Sypherd P. S. Protein synthesis during morphogenesis of Mucor racemosus. J Bacteriol. 1977 Oct;132(1):209–218. doi: 10.1128/jb.132.1.209-218.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznokas J. L., Sypherd P. S. Respiratory capacity, cyclic adenosine 3',5'-monophosphate, and morphogenesis of Mucor racemosus. J Bacteriol. 1975 Oct;124(1):134–139. doi: 10.1128/jb.124.1.134-139.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmit J. C., Brody S. Neurospora crassa conidial germination: role of endogenous amino acid pools. J Bacteriol. 1975 Oct;124(1):232–242. doi: 10.1128/jb.124.1.232-242.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele S. D., Miller J. J. Amino acid uptake and protein synthesis in germinating spores of Saccharomyces cerevisiae. Can J Microbiol. 1977 Apr;23(4):407–412. doi: 10.1139/m77-060. [DOI] [PubMed] [Google Scholar]
- Van Assche J. A., Carlier A. R. The pattern of protein and nucleic acid synthesis in germinating spores of Phycomyces blakesleeanus. Arch Mikrobiol. 1973 Oct 19;93(2):129–136. doi: 10.1007/BF00424943. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L. Protein synthesis during fungal spore germination. I. Characteristics of an in vitro phenylalanine incorporating system prepared from germinated spores of Botryodiplodia theobromae. Arch Biochem Biophys. 1968 Apr;125(1):13–21. doi: 10.1016/0003-9861(68)90632-2. [DOI] [PubMed] [Google Scholar]
- Yagura T., Iwabuchi M. DNA, RNA and protein synthesis during germination of spores in the cellular slime mold Dictyostelium discoideum. Exp Cell Res. 1976 Jun;100(1):79–87. doi: 10.1016/0014-4827(76)90329-3. [DOI] [PubMed] [Google Scholar]
- Young R., Bremer H. Polypeptide-chain-elongation rate in Escherichia coli B/r as a function of growth rate. Biochem J. 1976 Nov 15;160(2):185–194. doi: 10.1042/bj1600185. [DOI] [PMC free article] [PubMed] [Google Scholar]



