Abstract
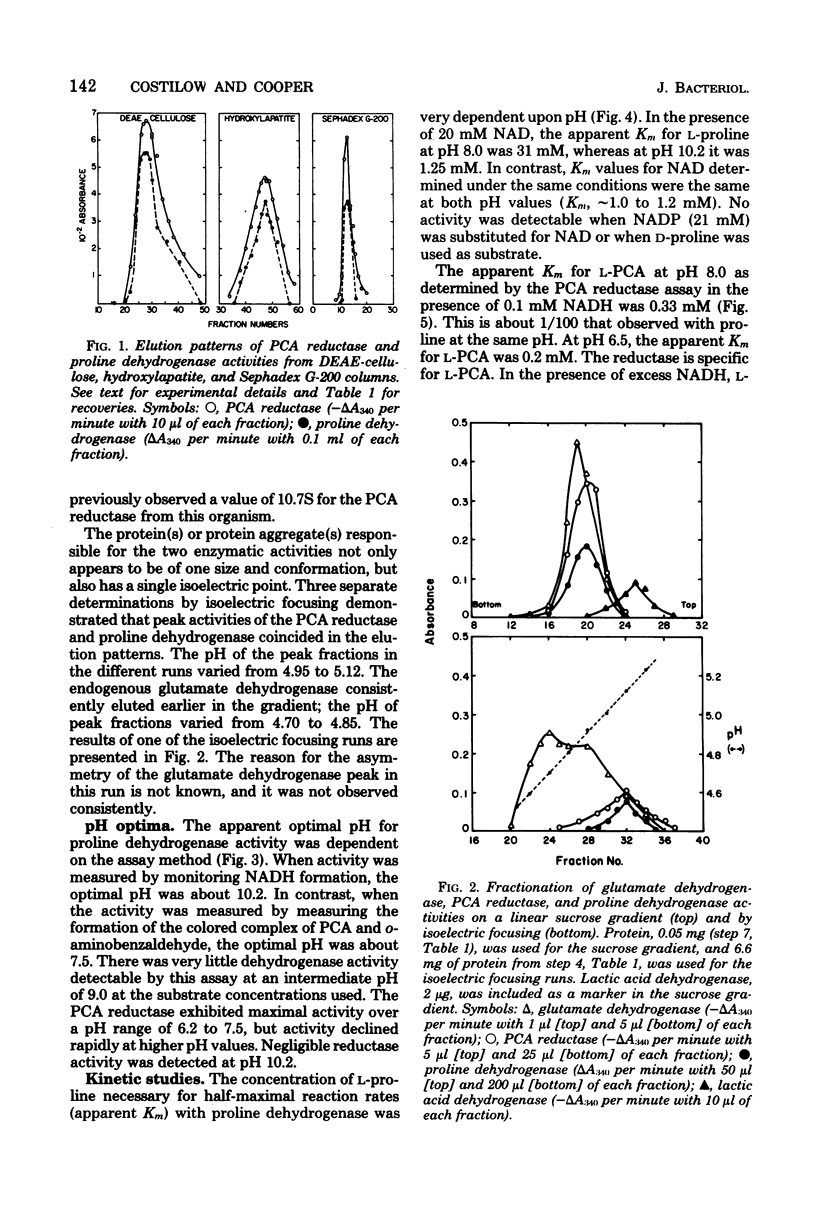
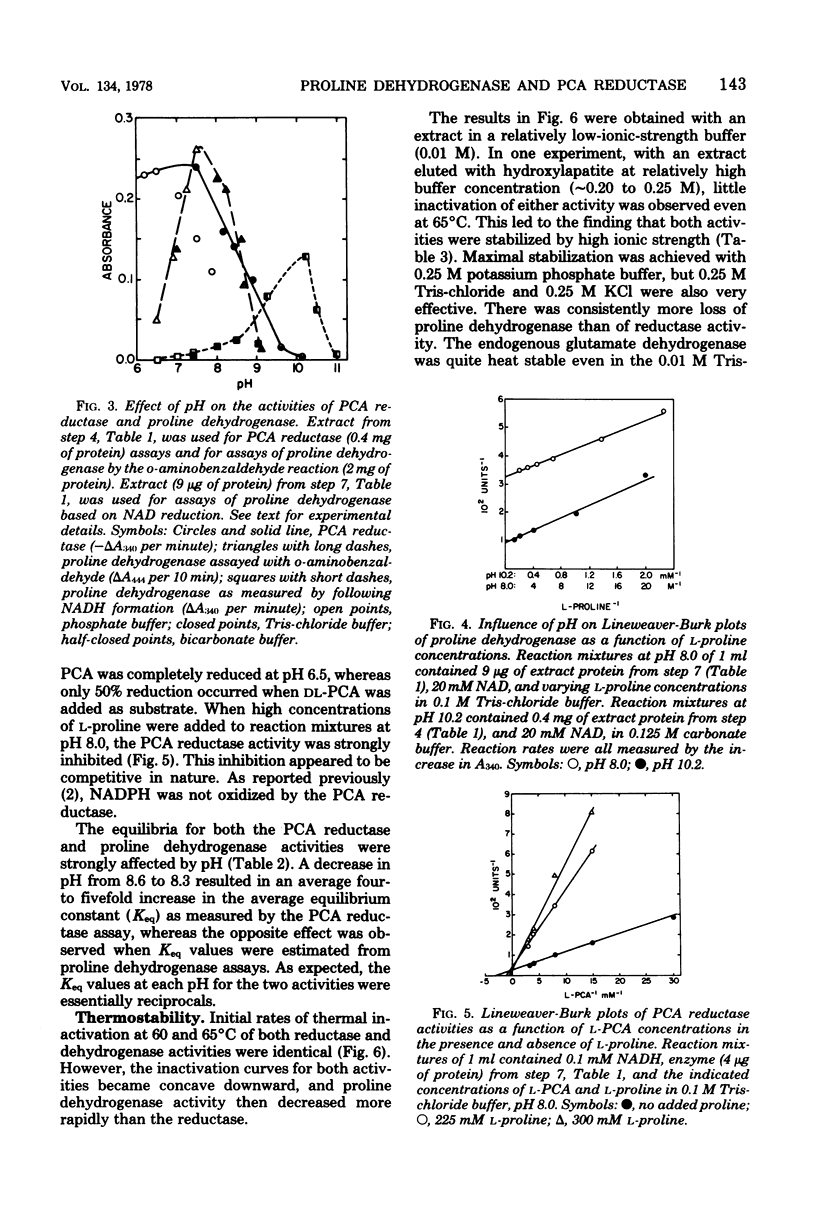
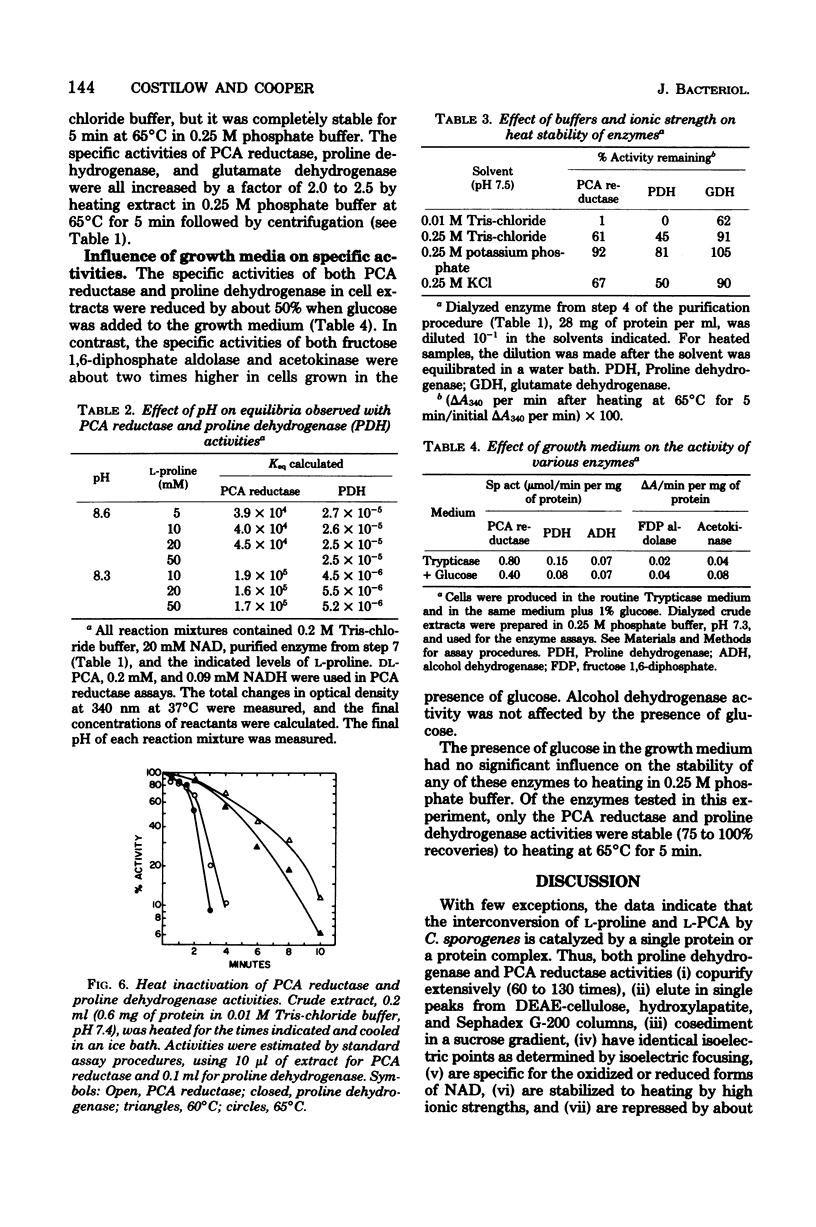
Proline dehydrogenase and delta1-pyrroline-5-carboxylic acid (PCA) reductase activities were copurified 60- and 130-fold, respectively, from extracts of Clostridium sporogenes. The primary change in the ratio of activites was the result of a loss of proline dehydrogenase activity during dialysis. Both activities were eluted in single peaks from diethylaminoethyl-cellulose, hydroxylapatite, and Sephadex G-200 columns. They had identical sedimentation coefficients (10.3S), as determined in linear sucrose gradients, and identical isoelectric points (4.95 to 5.12) based on isoelectric focusing. The proline dehydrogenase activity was dependent on nicotinamide adenine dinucleotide and L-proline, and the PCA reductase required L-PCA and reduced nicotinamide adenine dinucleotide. The optimum pH for the assay of proline dehydrogenase was approximately 10.2, whereas that for PCA reductase was 6.5 to 7.5. An increase in pH from 8.0 to 10.2 greatly decreased the apparent Michaelis constant observed for L-proline, and an increase from pH 8.3 to 8.6 resulted in a large shift in the reaction equilibrium toward PCA. Both the dehydrogenase and reductase activities were stabilized to heating at 65 degrees C for 5 min by solutes of high ionic strength and were inactivated in a similar fashion when dissolved in low-ionic-strength buffer. The specific activities for both were reduced by about 50% when glucose was added to the growth medium. The data support the conclusion that L-proline and L-PCA are interconverted by either a single enzyme or an enzyme complex in extracts of C. sporogenes cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Costilow R. N., Laycock L. Ornithine cyclase (deaminating). Purification of a protein that converts ornithine to proline and definition of the optimal assay conditions. J Biol Chem. 1971 Nov;246(21):6655–6660. [PubMed] [Google Scholar]
- Costilow R. N., Laycock L. Reactions involved in the conversion of ornithine to proline in Clostridia. J Bacteriol. 1969 Nov;100(2):662–667. doi: 10.1128/jb.100.2.662-667.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK L., RANHAND B. PROLINE METABOLISM IN ESCHERICHIA COLI. 3. THE PROLINE CATABOLIC PATHWAY. Arch Biochem Biophys. 1964 Aug;107:325–331. doi: 10.1016/0003-9861(64)90338-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MILLAR D. B. Physico-chemical properties of lactic dehydrogenase. J Biol Chem. 1962 Jul;237:2135–2139. [PubMed] [Google Scholar]
- McNamer A. D., Stewart C. R. Nicotinamide Adenine Dinucleotide-dependent Proline Dehydrogenase in Chlorella. Plant Physiol. 1974 Mar;53(3):440–444. doi: 10.1104/pp.53.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEISACH J., STRECKER H. J. The interconversion of glutamic acid and proline. V. The reduction of delta 1-pyrroline-5-carboxylic acid to proline. J Biol Chem. 1962 Jul;237:2255–2260. [PubMed] [Google Scholar]
- RACKER E. Crystalline alcohol dehydrogenase from baker's yeast. J Biol Chem. 1950 May;184(1):313–319. [PubMed] [Google Scholar]
- ROSE I. A., GRUNBERG-MANAGO M., KOREY S. R., OCHOA S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954 Dec;211(2):737–756. [PubMed] [Google Scholar]
- Rossi J. J., Vender J., Berg C. M., Coleman W. H. Partial purification and some properties of delta1-pyrroline-5-carboxylate reductase from Escherichia coli. J Bacteriol. 1977 Jan;129(1):108–114. doi: 10.1128/jb.129.1.108-114.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH M. E., GREENBERG D. M. Preparation and properties of partially purified glutamic semialdehyde reductase. J Biol Chem. 1957 May;226(1):317–327. [PubMed] [Google Scholar]
- Williams I., Frank L. Improved chemical synthesis and enzymatic assay of delta-1-pyrroline-5-carboxylic acid. Anal Biochem. 1975 Mar;64(1):85–97. doi: 10.1016/0003-2697(75)90408-x. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L., Barker H. A. Purification and properties of a NAD-dependent glutamate dehydrogenase from Clostridium SB4. Biochim Biophys Acta. 1970 Aug 15;212(2):225–242. doi: 10.1016/0005-2744(70)90203-2. [DOI] [PubMed] [Google Scholar]
- YURA T., VOGEL H. J. Pyrroline-5-carboxylate reductase of Neurospora crassa; partial purification and some properties. J Biol Chem. 1959 Feb;234(2):335–338. [PubMed] [Google Scholar]



