Abstract
The arginine pathway carbamoylphosphate synthase (CPSase A) from Saccharomyces cerevisiae was shown to be highly unstable and could not be substantially purified. In spite of this instability, a number of important properties of this enzyme were determined with crude preparations. A molecular weight of 140,000 (7.9S) was estimated for the native enzyme by sucrose gradient centrifugation; a significantly higher value, 175,000, was obtained by gel filtration on Sephadex. The enzyme is an aggregate consisting of two protein components, coded for by the unlinked genes cpaI and cpaII. These components were separated by diethylaminoethyl-cellulose chromatography. Their molecular weights, estimated by Sephadex gel filtration, were 36,000 and 130,000. The large component catalyzed the synthesis of carbamoylphosphate from ammonia. The small component was required in addition to the large one for the physiologically functional glutamine-dependent activity. Apparent Michaelis constants at pH 7.5 of 1.25 mM for glutamine and 75 mM for NH4Cl were measured with the native enzyme. The use of various glutamine analogs, including 2-amino-4-oxo-5-chloropentanoic acid, indicated that binding of glutamine to a site located on the small component was followed by transfer of its amide nitrogen to the ammonia site on the heavy component. This ammonia site was able to function independently of the utilization of glutamine. However, binding of glutamine was conjectured to cause a conformational change in the heavy component that greatly increased the rate of synthesis of carbamoylphosphate from ammonia. Glutamine, which was also shown to stabilize the aggregation of the two components, appeared to be a major effector of the catalytic and structural properties of CPSase A. In view of these observations, the CPSase A of yeast appears to share a number of structural and catalytic properties with the Escherichia coli enzyme. Obviously, the unlinked cpaI and cpaII genes of yeast are homologous to the adjacent carA and carB genes that code for the two subunits of the bacterial enzyme.
Full text
PDF


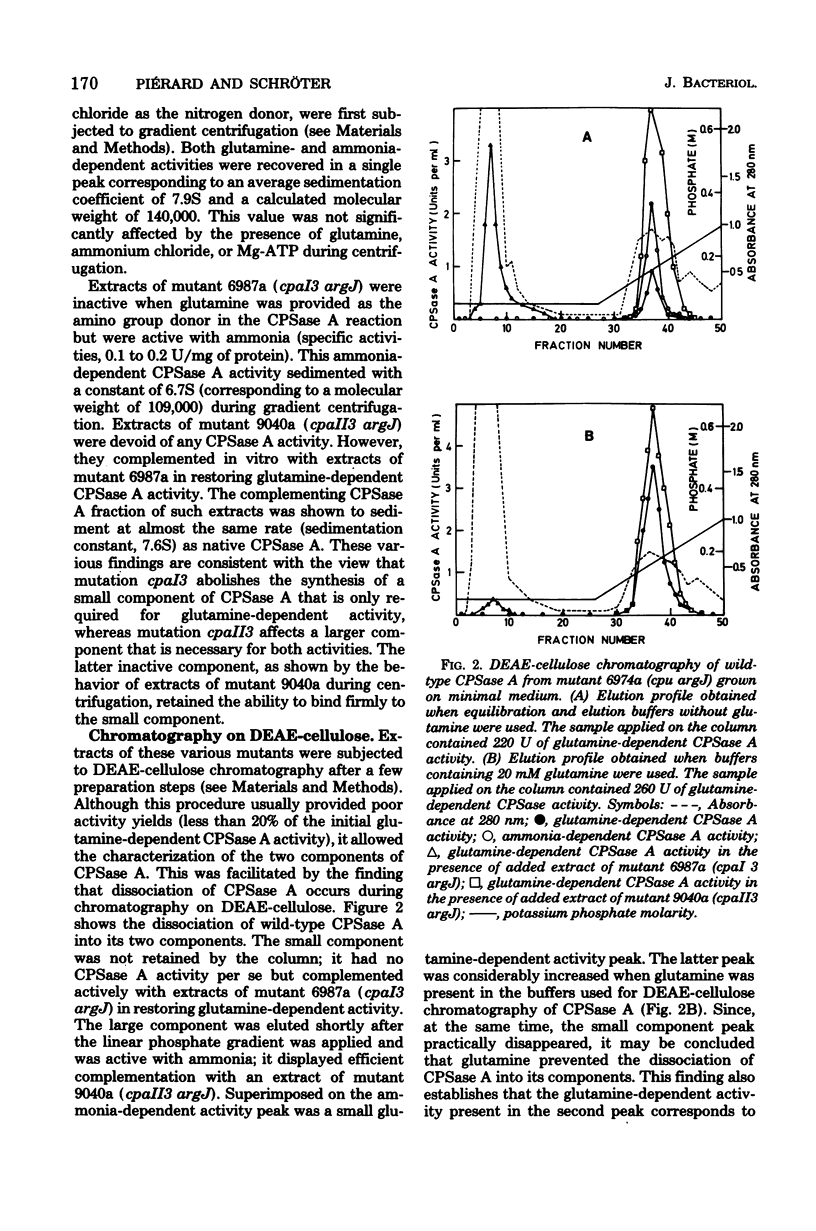
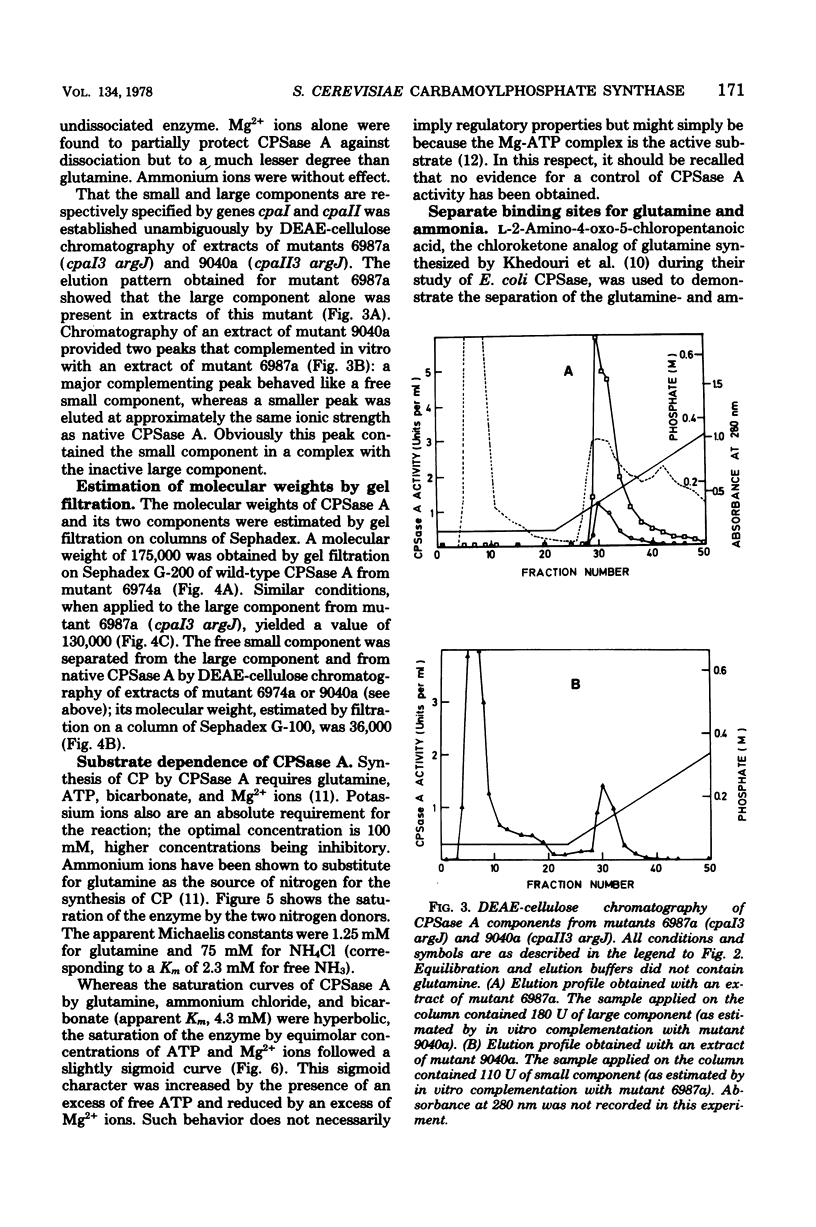
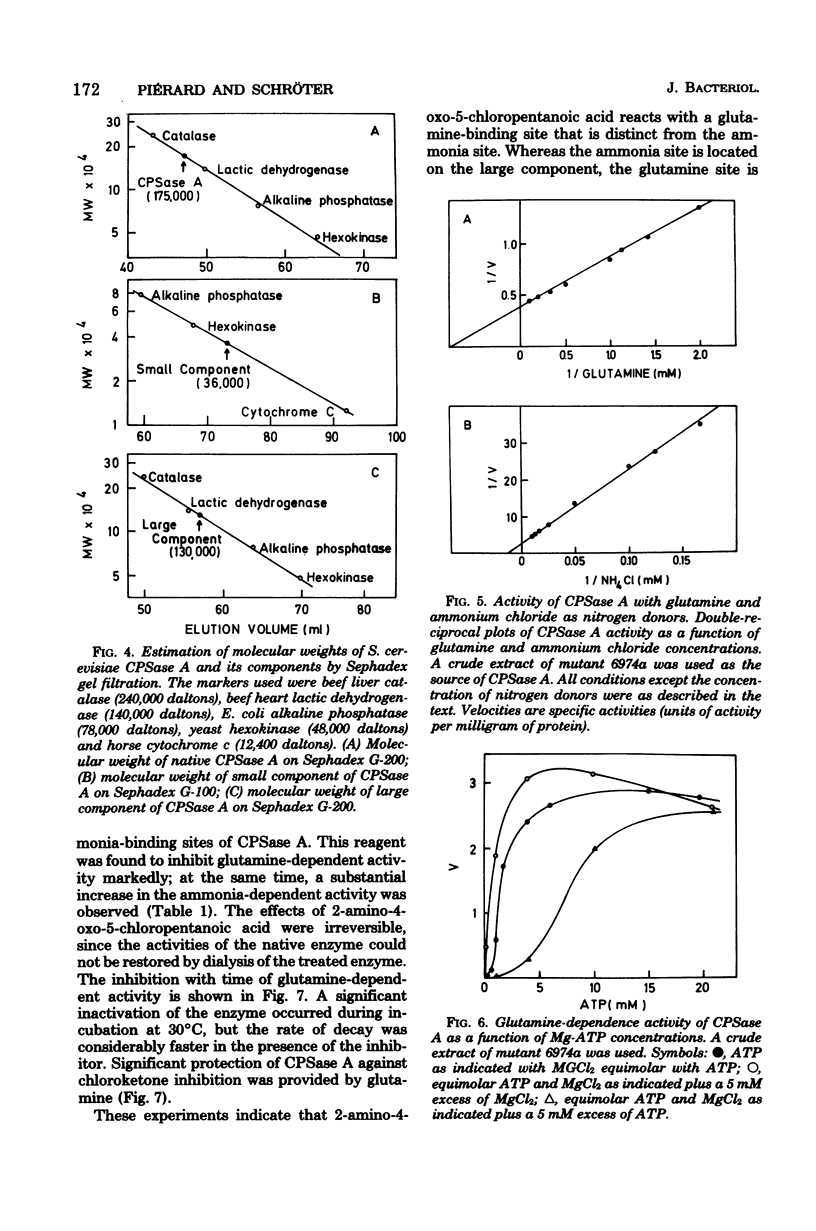
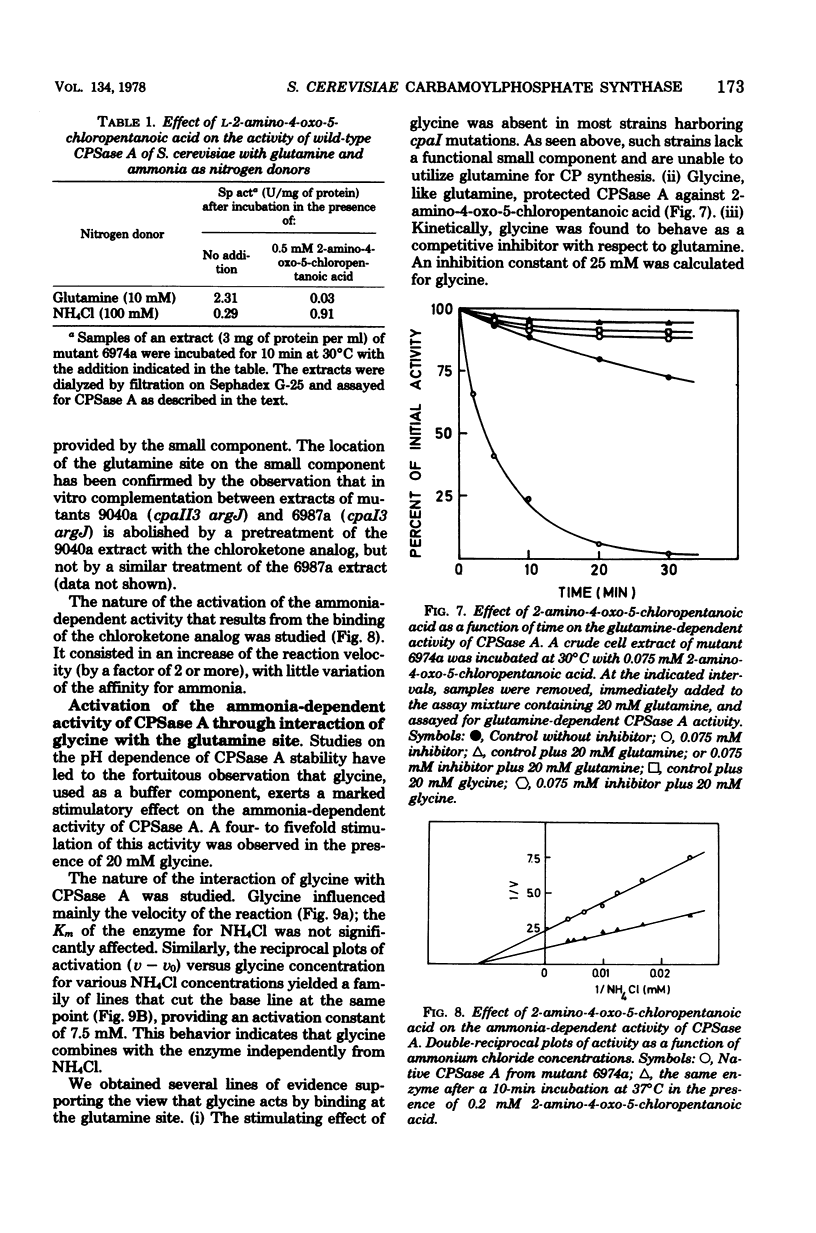


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T., Ingraham J. L. Carbamylphosphate synthetase from Salmonella typhimurium. Regulations, subunit composition, and function of the subunits. J Biol Chem. 1975 Jun 25;250(12):4410–4417. [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Mousset M., Wiame J. M., Bechet J. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. I. Evidence for a specific arginine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):325–338. doi: 10.1016/0304-4165(66)90387-4. [DOI] [PubMed] [Google Scholar]
- Kane J. F., Homes W. M., Smiley K. L., Jr, Jensen R. A. Rapid regulation of an anthranilate synthase aggregate by hysteresis. J Bacteriol. 1973 Jan;113(1):224–232. doi: 10.1128/jb.113.1.224-232.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedouri E., Anderson P. M., Meister A. Selective inactivation of the glutamine binding site of Escherichia coli carbamyl phosphate synthetase by 2-amino-4-oxo-5-chloropentanoic acid. Biochemistry. 1966 Nov;5(11):3552–3557. doi: 10.1021/bi00875a024. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacroute F., Piérard A., Grenson M., Wiame J. M. The biosynthesis of carbamoyl phosphate in Saccharomyces cerevisiae. J Gen Microbiol. 1965 Jul;40(1):127–142. doi: 10.1099/00221287-40-1-127. [DOI] [PubMed] [Google Scholar]
- London W. P., Steck T. L. Kinetics of enzyme reactions with interaction between a substrate and a (metal) modifier. Biochemistry. 1969 Apr;8(4):1767–1779. doi: 10.1021/bi00832a061. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Matthews S. L., Anderson P. M. Evidence for the presence of two nonidentical subunits in carbamyl phosphate synthetase of Escherichia coli. Biochemistry. 1972 Mar 28;11(7):1176–1183. doi: 10.1021/bi00757a010. [DOI] [PubMed] [Google Scholar]
- Mergeay M., Gigot D., Beckmann J., Glansdorff N., Piérard A. Physiology and genetics of carbamoylphosphate synthesis in Escherichia coli K12. Mol Gen Genet. 1974;133(4):299–316. doi: 10.1007/BF00332706. [DOI] [PubMed] [Google Scholar]
- Pinkus L. M., Meister A. Identification of a reactive cysteine residue at the glutamine binding site of carbamyl phosphate synthetase. J Biol Chem. 1972 Oct 10;247(19):6119–6127. [PubMed] [Google Scholar]
- Queener S. W., Queener S. F., Meeks J. R., Gunsalus I. C. Anthranilate synthase from Pseudomonas putida. Purification and properties of a two-component enzyme. J Biol Chem. 1973 Jan 10;248(1):151–161. [PubMed] [Google Scholar]
- Ramos F., Thuriaux P., Wiame J. M., Bechet J. The participation of ornithine and citrulline in the regulation of arginine metabolism in Saccharomyces cerevisiae. Eur J Biochem. 1970 Jan;12(1):40–47. doi: 10.1111/j.1432-1033.1970.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Sachs D. H., Painter E. Improved flow rates with porous sephadex gels. Science. 1972 Feb 18;175(4023):781–782. doi: 10.1126/science.175.4023.781. [DOI] [PubMed] [Google Scholar]
- Thuriaux P., Ramos F., Piérard A., Grenson M., Wiame J. M. Regulation of the carbamoylphosphate synthetase belonging to the arginine biosynthetic pathway of Saccharomyces cerevisiae. J Mol Biol. 1972 Jun 20;67(2):277–287. doi: 10.1016/0022-2836(72)90241-0. [DOI] [PubMed] [Google Scholar]
- Trotta P. P., Pinkus L. M., Haschemeyer R. H., Meister A. Reversible dissociation of the monomer of glutamine-dependent carbamyl phosphate synthetase into catalytically active heavy and light subunits. J Biol Chem. 1974 Jan 25;249(2):492–499. [PubMed] [Google Scholar]
- Urrestarazu L. A., Vissers S., Wiame J. M. Change in location of ornithine carbamoyltransferase and carbamoylphosphate synthetase among yeasts in relation to the arginase/ornithine carbamoyltransferase regulatory complex and the energy status of the cells. Eur J Biochem. 1977 Oct 3;79(2):473–481. doi: 10.1111/j.1432-1033.1977.tb11830.x. [DOI] [PubMed] [Google Scholar]
- Wellner V. P., Anderson P. M., Meister A. Interaction of Escherichia coli carbamyl phosphate synthetase with glutamine. Biochemistry. 1973 May 22;12(11):2061–2066. doi: 10.1021/bi00735a006. [DOI] [PubMed] [Google Scholar]
- Wellner V. P., Meister A. Enhancement of the glutaminase activity of carbamyl phosphate synthetase by alterations in the interaction between the heavy and light subunits. J Biol Chem. 1975 May 10;250(9):3261–3266. [PubMed] [Google Scholar]



