Abstract
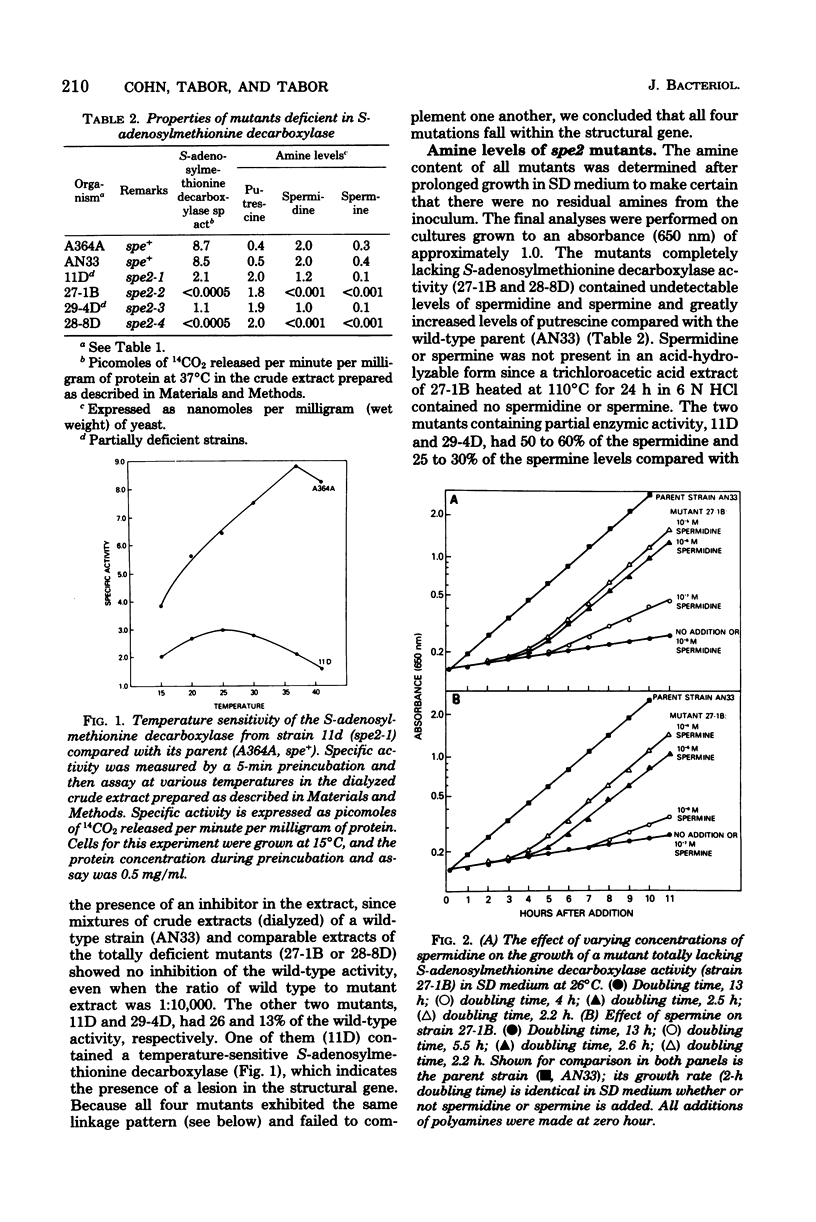
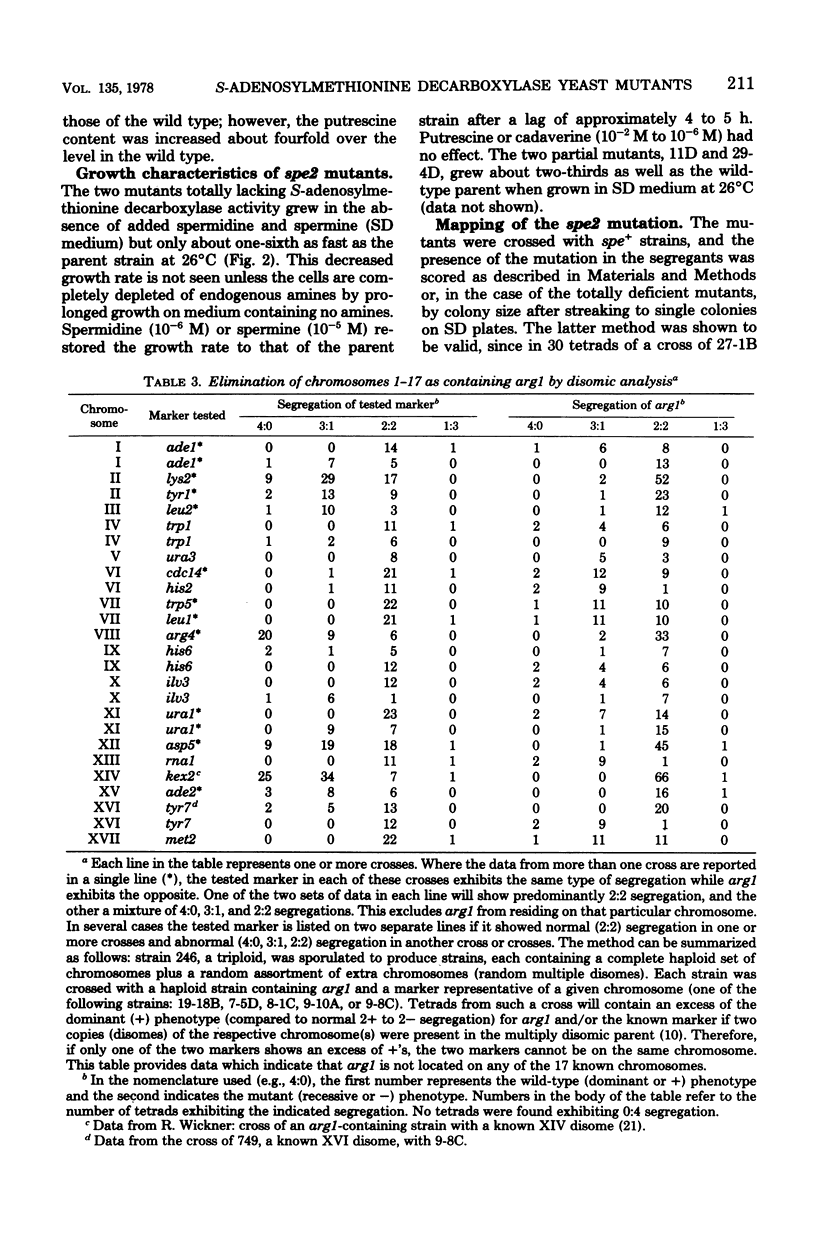
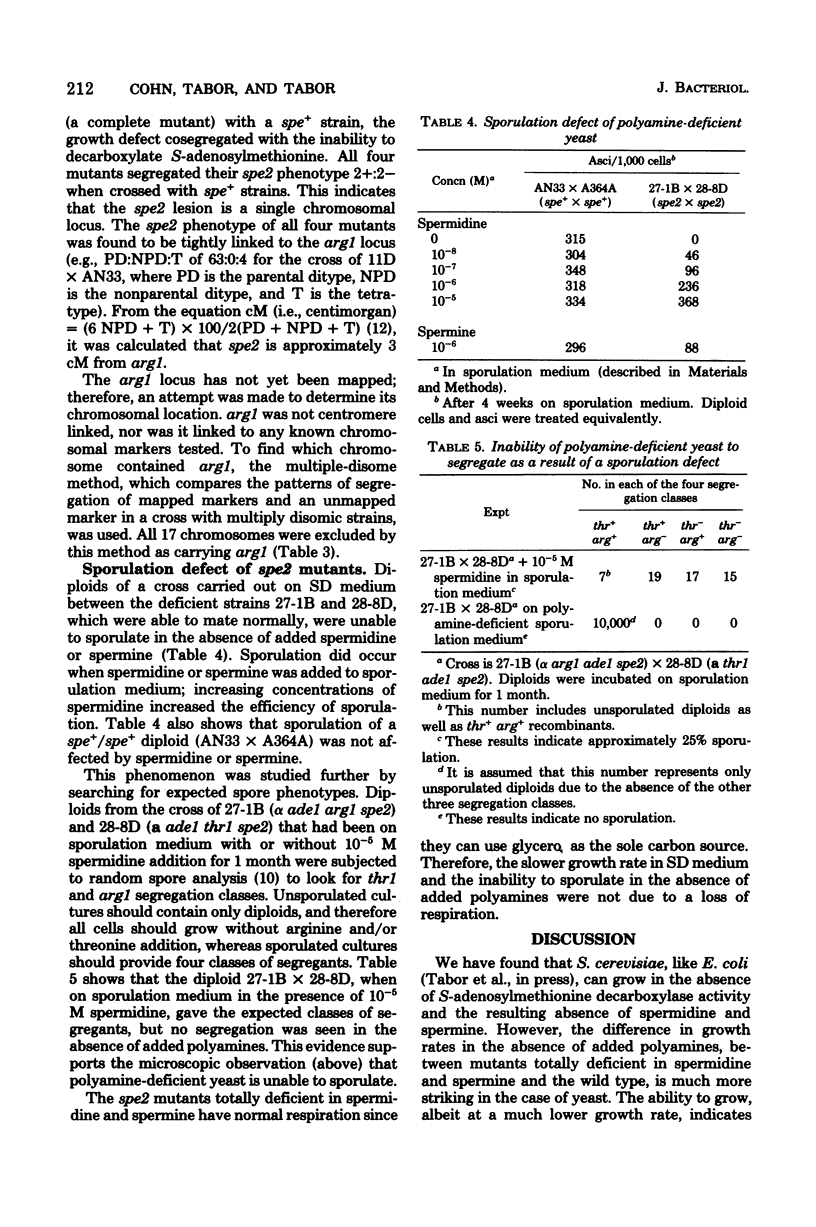
Four mutants were isolated from Saccharomyces cerevisiae that are deficient in S-adenosylmethionine decarboxylase (spe2). All four mutants are chromosomal and fall into a single complementation group tightly linked to arg1. Since one of the mutants contained a temperature-sensitive activity, this complementation group defines the structural gene. Mutants totally lacking enzymic activity did not contain spermidine or spermine and had a greatly increased doubling time when grown in the absence of these two polyamines. Addition of 10(-6) M spermidine or 10(-5) M spermine, but not putrescine or cadaverine, restored the doubling time to that of the wild type. Diploids formed from a cross of two mutants completely deficient in spermidine and spermine were unable to sporulate in the absence of added spermidine or spermine. We obtained evidence that arg1 was not located on any of the 17 known chromosomes, and therefore we postulate that arg1 and spe2 are located on a new 18th chromosome.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohn M. S., Tabor C. W., Tabor H. Identification of a pyruvoyl residue in S-adenosylmethionine decarboxylase from Saccharomyces cerevisiae. J Biol Chem. 1977 Nov 25;252(22):8212–8216. [PubMed] [Google Scholar]
- Cunningham-Rundles S., Maas W. K. Isolation, characterization, and mapping of Escherichia coli mutants blocked in the synthesis of ornithine decarboxylase. J Bacteriol. 1975 Nov;124(2):791–799. doi: 10.1128/jb.124.2.791-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou A. A., Cohn M. S., Tabor C. W., Tabor H. Identification of pyruvate in S-adenosylmethionine decarboxylase from rat liver. J Biol Chem. 1978 Mar 10;253(5):1684–1686. [PubMed] [Google Scholar]
- Groves W. E., Davis F. C., Jr, Sells B. H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968 Feb;22(2):195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- Janne J., Williams-Ashman H. G., Schenone A. Spermidine synthesizing enzymes in baker's yeast. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1362–1368. doi: 10.1016/s0006-291x(71)80024-4. [DOI] [PubMed] [Google Scholar]
- Kee S. G., Haber J. E. Cell cycle-dependent induction of mutations along a yeast chromosome. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1179–1183. doi: 10.1073/pnas.72.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Kaneko T., Yamamoto Y. Lysis of viable yeast cells by enzymes of Arthrobacter luteus. Arch Biochem Biophys. 1971 Jul;145(1):402–404. doi: 10.1016/0003-9861(71)90053-1. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic Mapping in Saccharomyces IV. Mapping of Temperature-Sensitive Genes and Use of Disomic Strains in Localizing Genes. Genetics. 1973 May;74(1):33–54. doi: 10.1093/genetics/74.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. Purification of rat liver S-adenosyl-L-methionine decarboxylase. Biochem J. 1974 Aug;141(2):581–583. doi: 10.1042/bj1410581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. D. Biochemical Mutants in the Smut Fungus Ustilago Maydis. Genetics. 1949 Sep;34(5):607–626. doi: 10.1093/genetics/34.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABOR H., ROSENTHAL S. M., TABOR C. W. The biosynthesis of spermidine and spermine from putrescine and methionine. J Biol Chem. 1958 Oct;233(4):907–914. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W., Irreverre F. Quantitative determination of aliphatic diamines and polyamines by an automated liquid chromatography procedure. Anal Biochem. 1973 Oct;55(2):457–467. doi: 10.1016/0003-2697(73)90136-x. [DOI] [PubMed] [Google Scholar]
- Weiss B., Milcarek C. Mass screening for mutants with altered DNases by microassay techniques. Methods Enzymol. 1974;29:180–193. doi: 10.1016/0076-6879(74)29020-7. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Chromosomal and nonchromosomal mutations affecting the "killer character" of Saccharomyces cerevisiae. Genetics. 1974 Mar;76(3):423–432. doi: 10.1093/genetics/76.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Two chromosomal genes required for killing expression in killer strains of Saccharomyces cerevisiae. Genetics. 1976 Mar 25;82(3):429–442. doi: 10.1093/genetics/82.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Tabor C. W., Tabor H. Purification of adenosylmethionine decarboxylase from Escherichia coli W: evidence for covalently bound pyruvate. J Biol Chem. 1970 Apr 25;245(8):2132–2139. [PubMed] [Google Scholar]



