Abstract
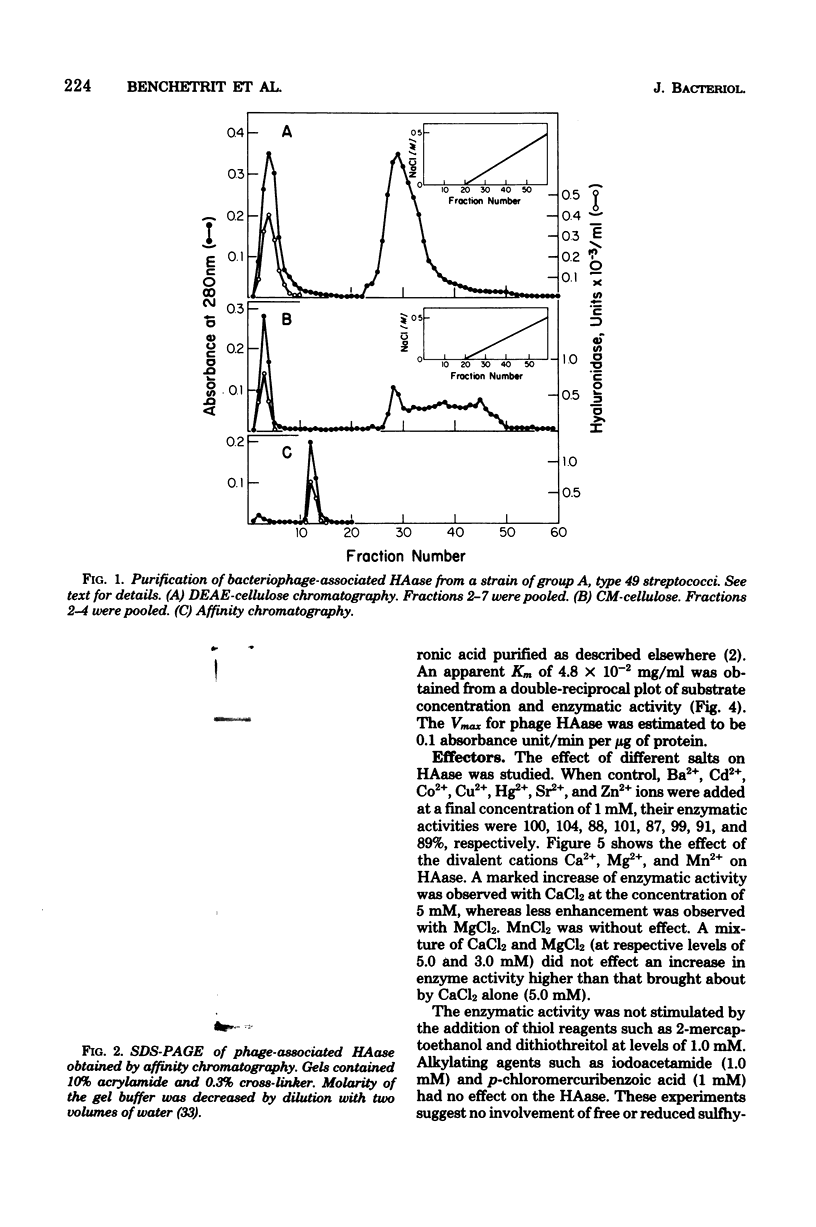
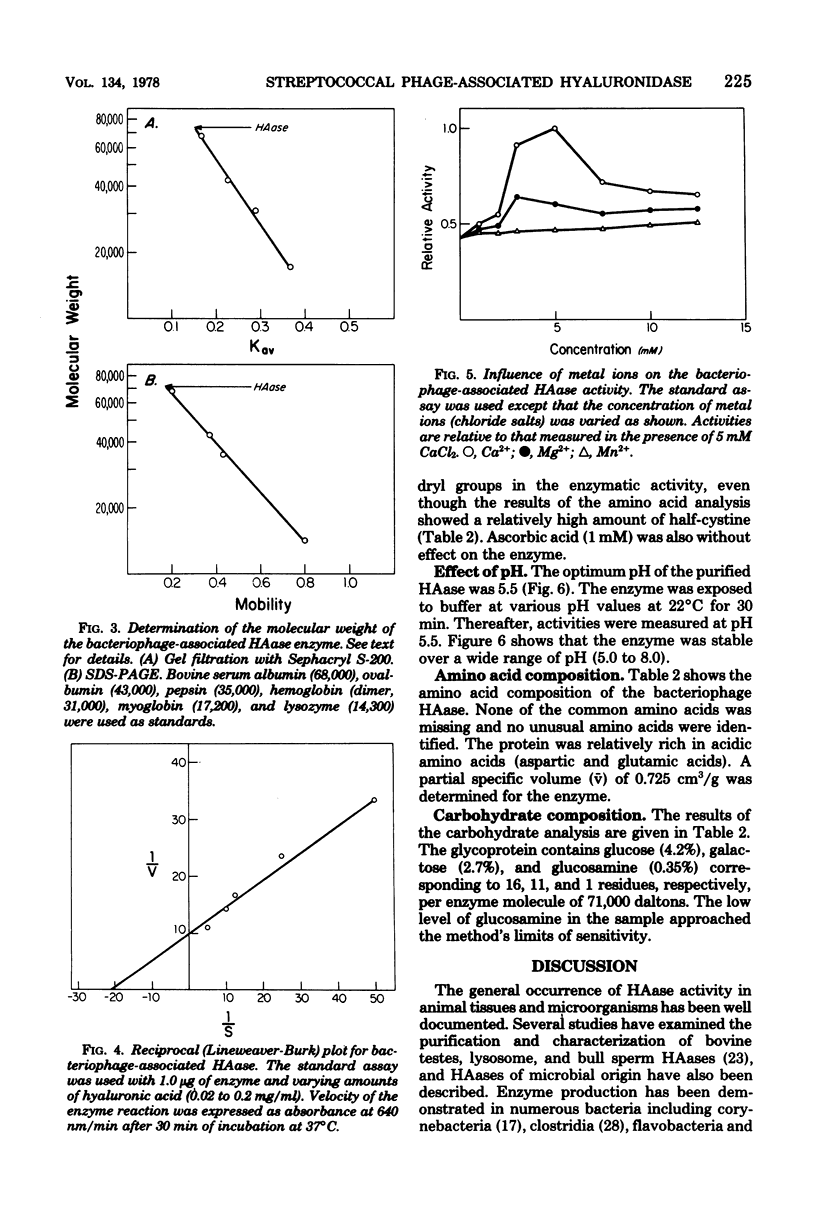
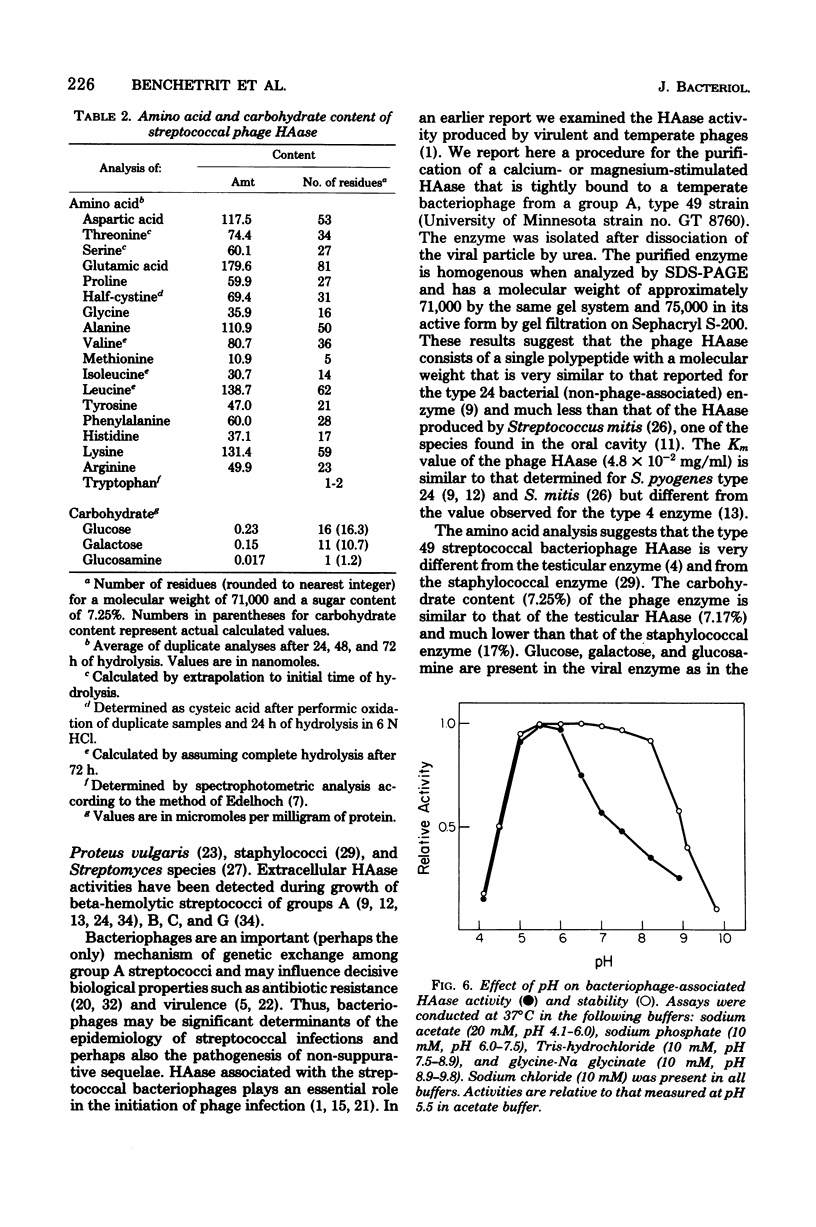
Urea treatment of a temperate bacteriophage from a type 49 strain of group A streptococcus (Streptococcus pyogenes) followed by ammonium sulfate fractionation, ion exchange, and affinity chromatography of solubilized proteins provided for the recovery (12%) and purification (44-fold) of the phage-associated hyaluronidase. The molecular weight of the homogeneous, purified enzyme was estimated to be 71,000 by polyacrylamide gel electrophoresis (in the presence of sodium dodecyl sulfate) and 75,000 by gel filtration with Sephacryl S-200. The enzyme has a pH optimum of 5.5, a Vmax of 0.1 absorbance unit/min per microgram of protein, and a Km of 4.8 X 10(-2) mg/ml with umbilical cord hyaluronic acid as substrate. Of the cations tested, calcium and magnesium were the only effectors of the enzyme. The enzyme is a glycoprotein (7.25% carbohydrate) containing glucose, galactose, and glucosamine. Analysis of the amino acid composition revealed a predominance of acidic amino acids and a relatively high content of cysteine. The partial specific volume, estimated from the amino acid and sugar analyses, was 0.725 cm3/g.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benchetrit L. C., Gray E. D., Wannamaker L. W. Hyaluronidase activity of bacteriophages of group A streptococci. Infect Immun. 1977 Feb;15(2):527–532. doi: 10.1128/iai.15.2.527-532.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchetrit L. C., Pahuja S. L., Gray E. D., Edstrom R. D. A sensitive method for the assay of hyaluronidase activity. Anal Biochem. 1977 May 1;79(1-2):431–437. doi: 10.1016/0003-2697(77)90418-3. [DOI] [PubMed] [Google Scholar]
- Bhatti T., Chambers R. E., Clamp J. R. The gas chromatographic properties of biologically important N-acetylglucosamine derivatives, monosaccharides, disaccharides, trisaccharides, tetrasaccharides and pentasaccharides. Biochim Biophys Acta. 1970 Nov 24;222(2):339–347. doi: 10.1016/0304-4165(70)90122-4. [DOI] [PubMed] [Google Scholar]
- Borders C. L., Jr, Raftery M. A. Purification and partial characterization of testicular hyaluronidase. J Biol Chem. 1968 Jul 10;243(13):3756–3762. [PubMed] [Google Scholar]
- Cleary P. P., Johnson Z. Possible dual function of M protein: resistance to bacteriophage A25 and resistance to phagocytosis by human leukocytes. Infect Immun. 1977 Apr;16(1):280–292. doi: 10.1128/iai.16.1.280-292.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Edstrom R. D. A colorimetric method for the determination of mucopolysaccharides and other acidic polymers. Anal Biochem. 1969 Jun;29(3):421–432. doi: 10.1016/0003-2697(69)90327-3. [DOI] [PubMed] [Google Scholar]
- GREILING H., STUHLSATZ H. W., EBERHARD T. ZUR HETEROGENITAET DER HYALURONATLYASE. Hoppe Seylers Z Physiol Chem. 1965;340:243–248. doi: 10.1515/bchm2.1965.340.1-2.243. [DOI] [PubMed] [Google Scholar]
- Gerlach D., Köhler W. Hyaluronatlyase von Streptococcus pyogenes. II. Charakterisierung der Hyaluronatlyase. Zentralbl Bakteriol Orig A. 1972 Aug;221(3):296–302. [PubMed] [Google Scholar]
- Hill J. Purification and properties of streptococcal hyaluronate lyase. Infect Immun. 1976 Sep;14(3):726–735. doi: 10.1128/iai.14.3.726-735.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard I. K., Sage H. J., Stein M. D., Young N. M., Leon M. A., Dyckes D. F. Studies on a phytohemagglutinin from the lentil. II. Multiple forms of Lens culinaris hemagglutinin. J Biol Chem. 1971 Mar 25;246(6):1590–1595. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linder L., Nord C. E., Söder P. O. Separation of hyaluronidase from a strain of anaerobic corynebacteria. Scand J Dent Res. 1971;79(7):528–532. doi: 10.1111/j.1600-0722.1971.tb02051.x. [DOI] [PubMed] [Google Scholar]
- MAXTED W. R. Enhancement of streptococcal bacteriophage lysis by hyaluronidase. Nature. 1952 Dec 13;170(4337):1020–1021. doi: 10.1038/1701020b0. [DOI] [PubMed] [Google Scholar]
- MAXTED W. R. The influence of bacteriophage on Streptococcus pyogenes. J Gen Microbiol. 1955 Jun;12(3):484–495. doi: 10.1099/00221287-12-3-484. [DOI] [PubMed] [Google Scholar]
- MOGILEVSKII M. S. ISOLATION AND PURIFICATION OF STREPTOCOCCAL HYALURONIDASE. Fed Proc Transl Suppl. 1964 May-Jun;23:559–561. [PubMed] [Google Scholar]
- Malke H., Starke R., Köhler W., Kolesnichenko G., Totolian A. A. Bacteriophage P13234mo-mediated intra- and intergroup transduction of antibiotic resistance among streptococci. Zentralbl Bakteriol Orig A. 1975 Sep;233(1):24–34. [PubMed] [Google Scholar]
- Niemann H., Birch-Andersen A., Kjems E., Mansa B., Stirm S. Streptococcal bacteriophage 12/12-borne hyaluronidase and its characterization as a lyase (EC 4.2.99.1) by means of streptococcal hyaluronic acid and purified bacteriophage suspensions. Acta Pathol Microbiol Scand B. 1976 Jun;84(3):145–153. doi: 10.1111/j.1699-0463.1976.tb01917.x. [DOI] [PubMed] [Google Scholar]
- Nord C. E. Purification and properties of hyaluronidase from Streptococcus mitis. Odontol Revy. 1971;22(2):125–136. [PubMed] [Google Scholar]
- Ohya T., Kaneko Y. Novel hyaluronidase from streptomyces. Biochim Biophys Acta. 1970 Mar 18;198(3):607–609. doi: 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- Princewill T. J., Oakley C. L. The deoxyribonucleases and hyaluronidases of Clostridium septicum and Cl. chauvoei. II. An agar plate method for testing for hyaluronidase. Med Lab Technol. 1972 Jul;29(3):255–260. [PubMed] [Google Scholar]
- Rautela G. S., Abramson C. Crystallization and partial characterization of Staphylococcus aureus hyaluronate lyase. Arch Biochem Biophys. 1973 Oct;158(2):687–694. doi: 10.1016/0003-9861(73)90562-6. [DOI] [PubMed] [Google Scholar]
- WENNER H. A., GIBSON D. M., JAQUES R. Specificities of hyaluronidases formed by several groups of streptococci. Proc Soc Exp Biol Med. 1951 Mar;76(3):585–587. doi: 10.3181/00379727-76-18567. [DOI] [PubMed] [Google Scholar]
- Wannamaker L. W., Skjold S., Maxted W. R. Characterization of bacteriophages from nephritogenic group A streptococci. J Infect Dis. 1970 Apr;121(4):407–418. doi: 10.1093/infdis/121.4.407. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yang C. H., Srivastava P. N. Purification of bull sperm hyaluronidase by concanavalin-A affinity chromatography. Biochim Biophys Acta. 1975 Jun 24;391(2):382–387. doi: 10.1016/0005-2744(75)90261-2. [DOI] [PubMed] [Google Scholar]




