Abstract
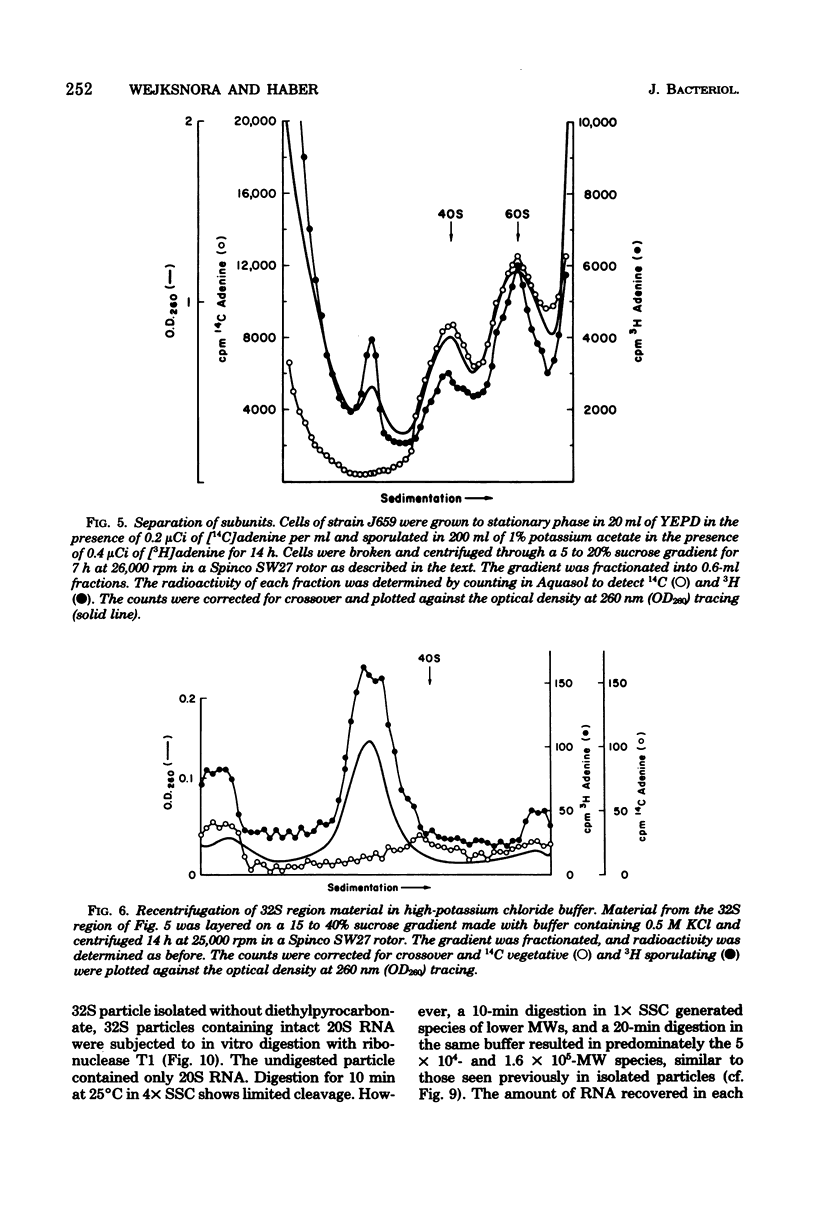
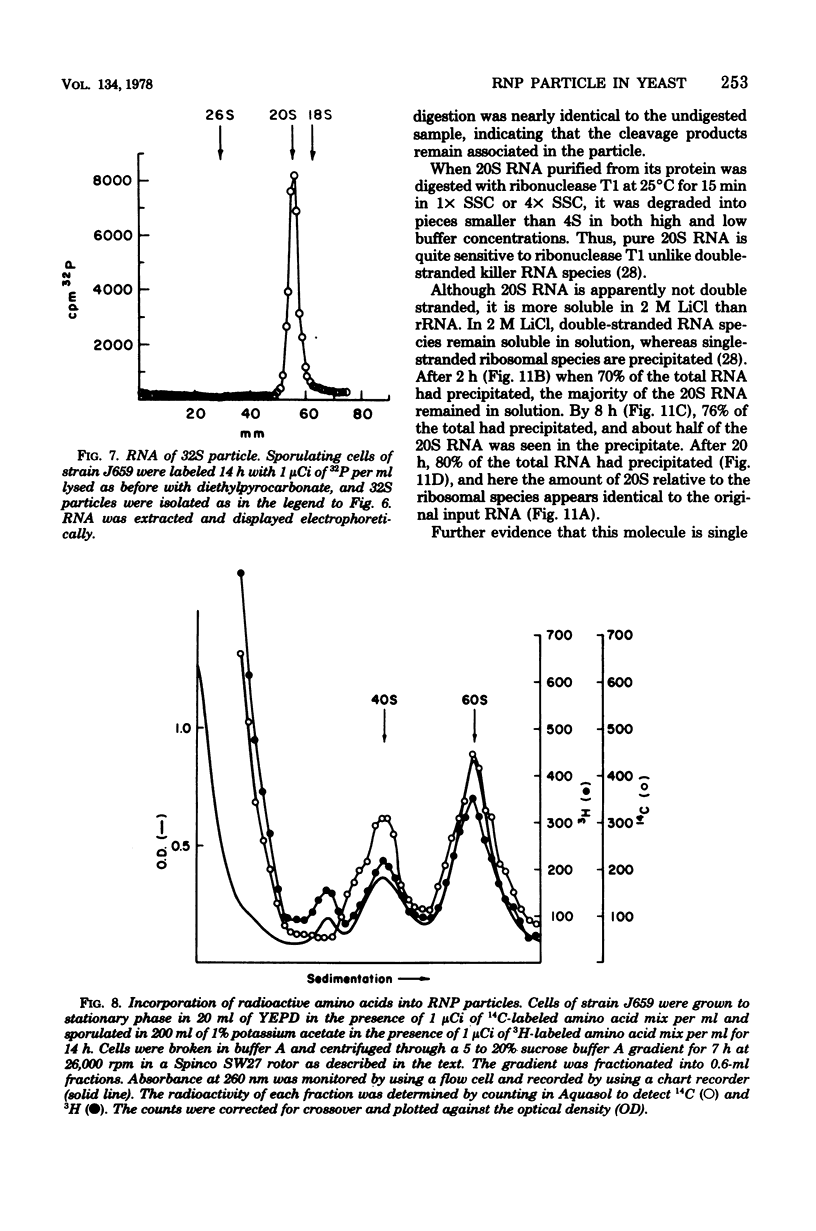
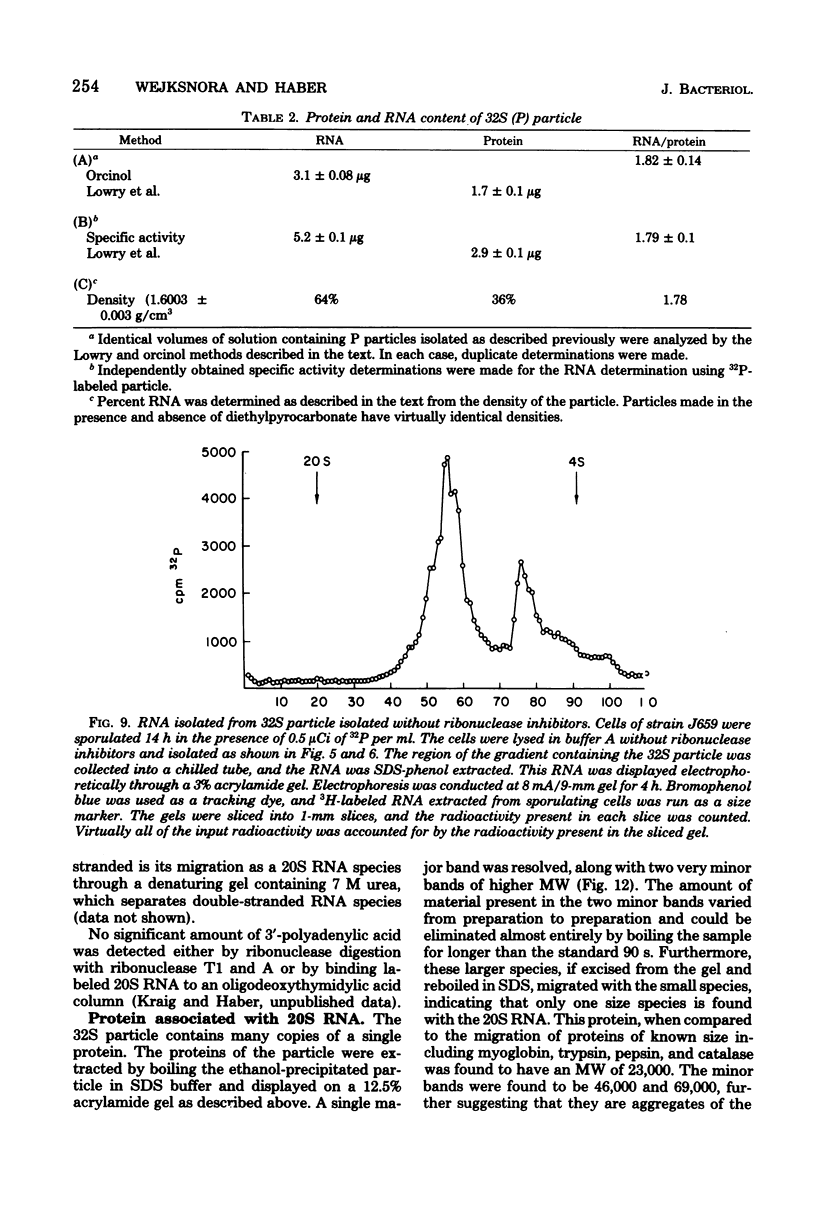
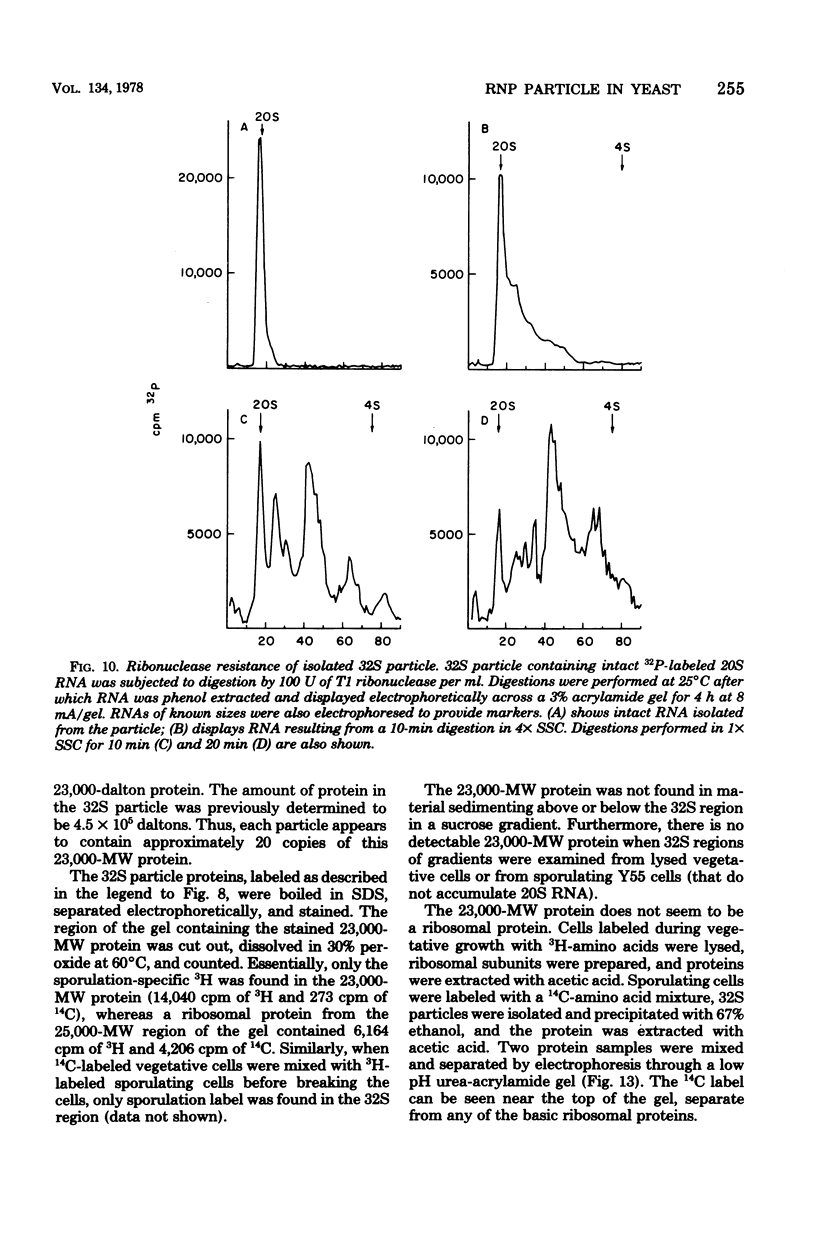
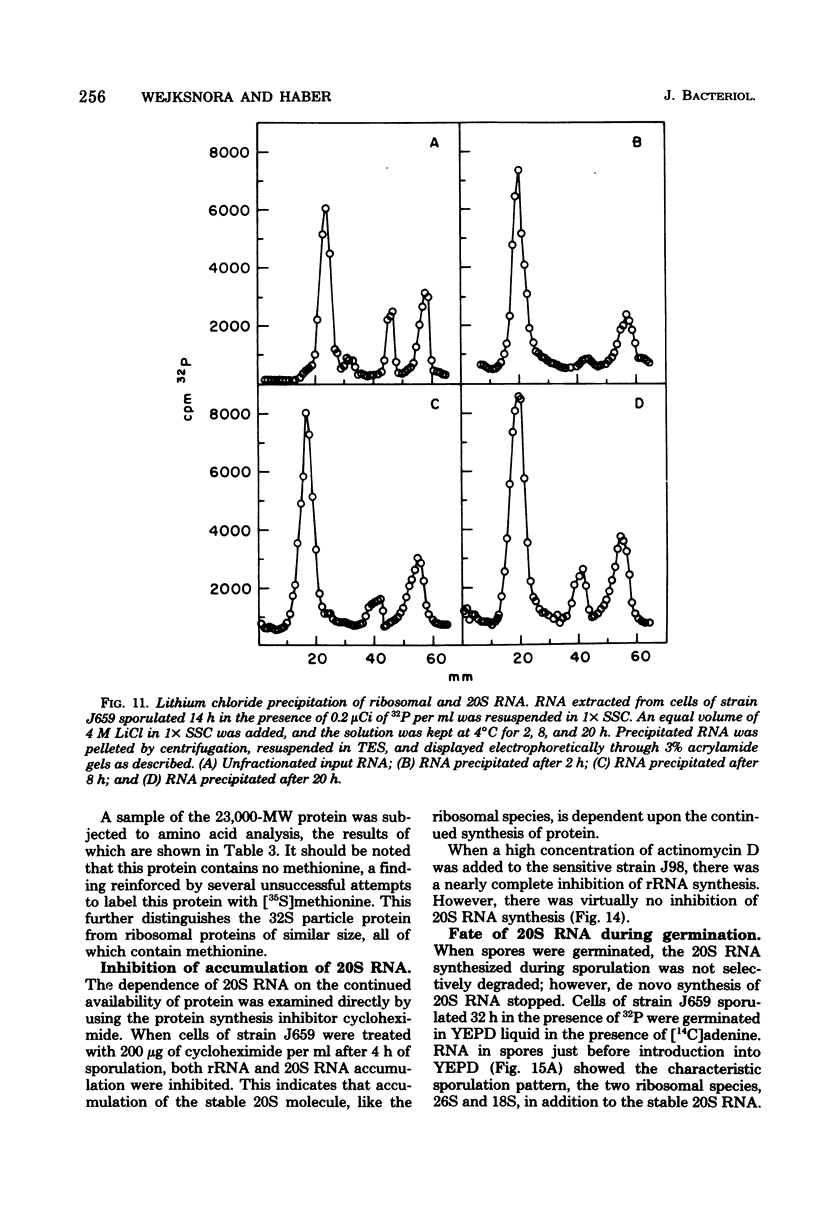
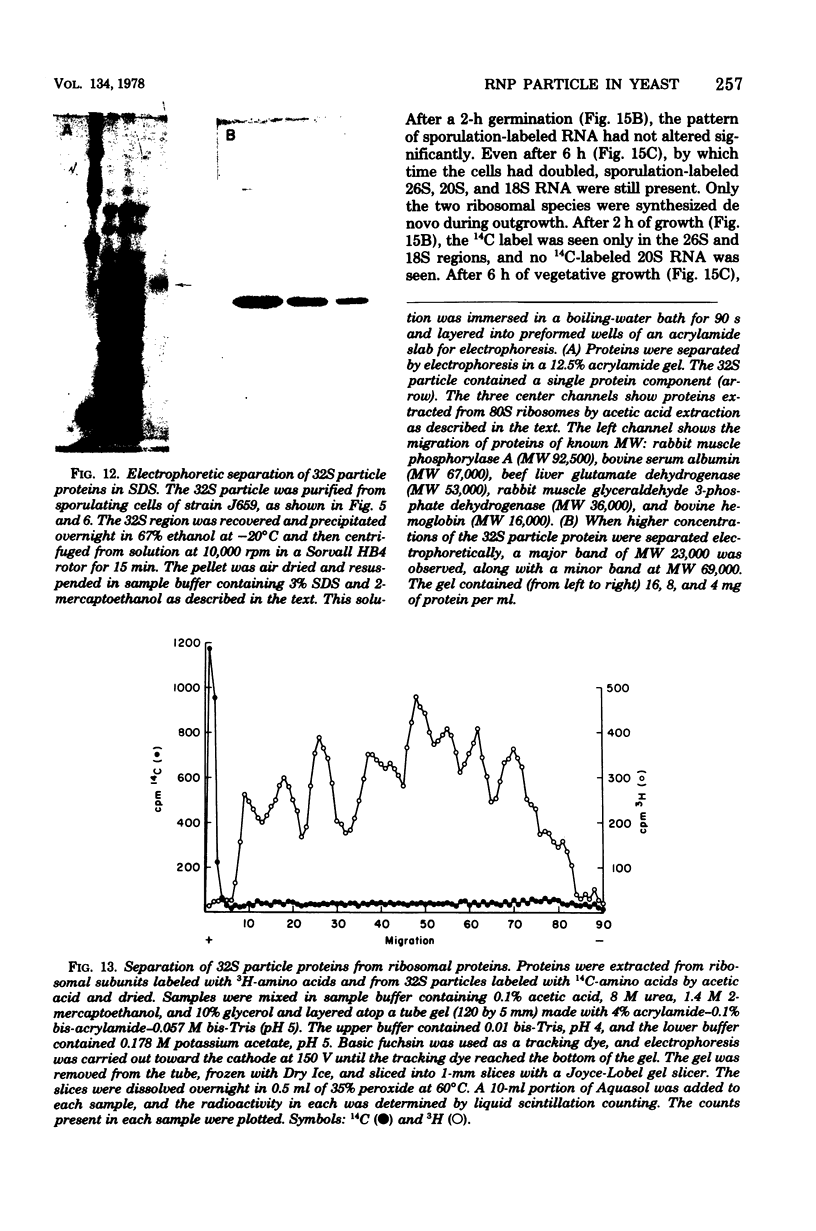
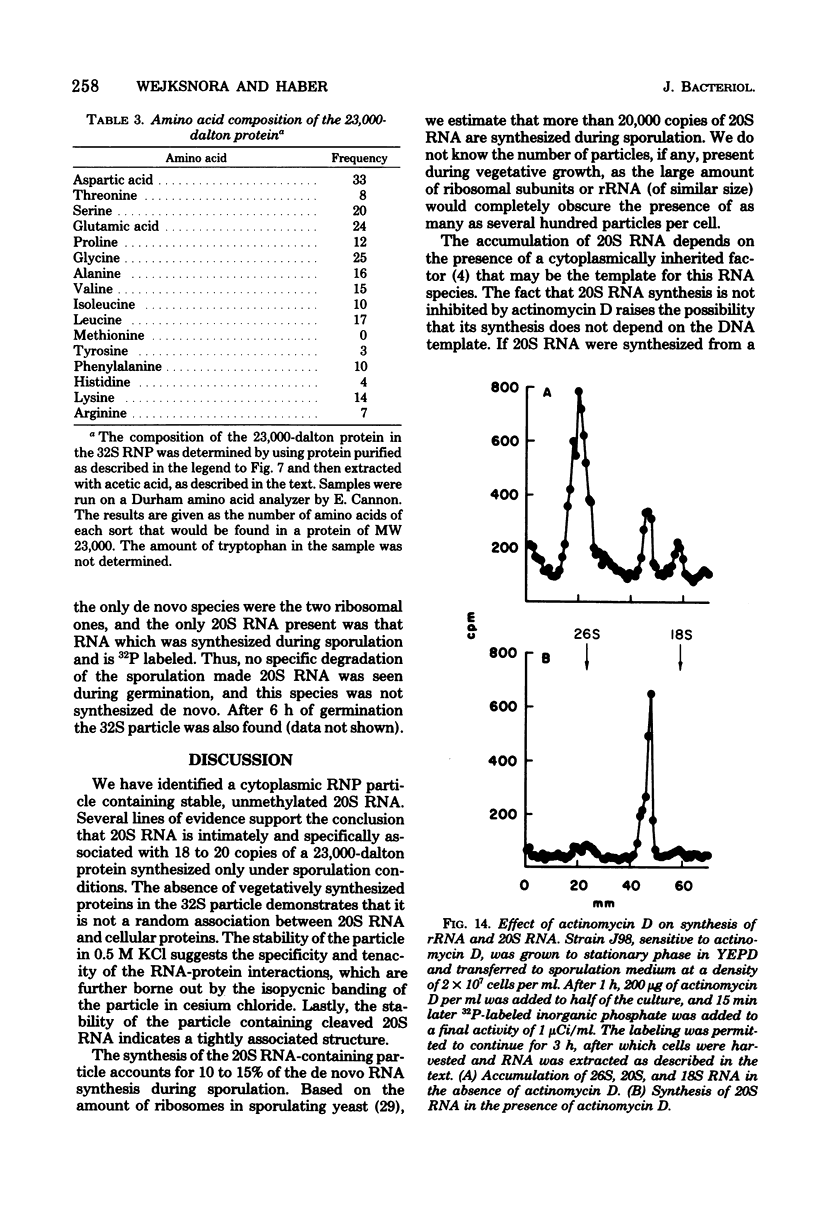
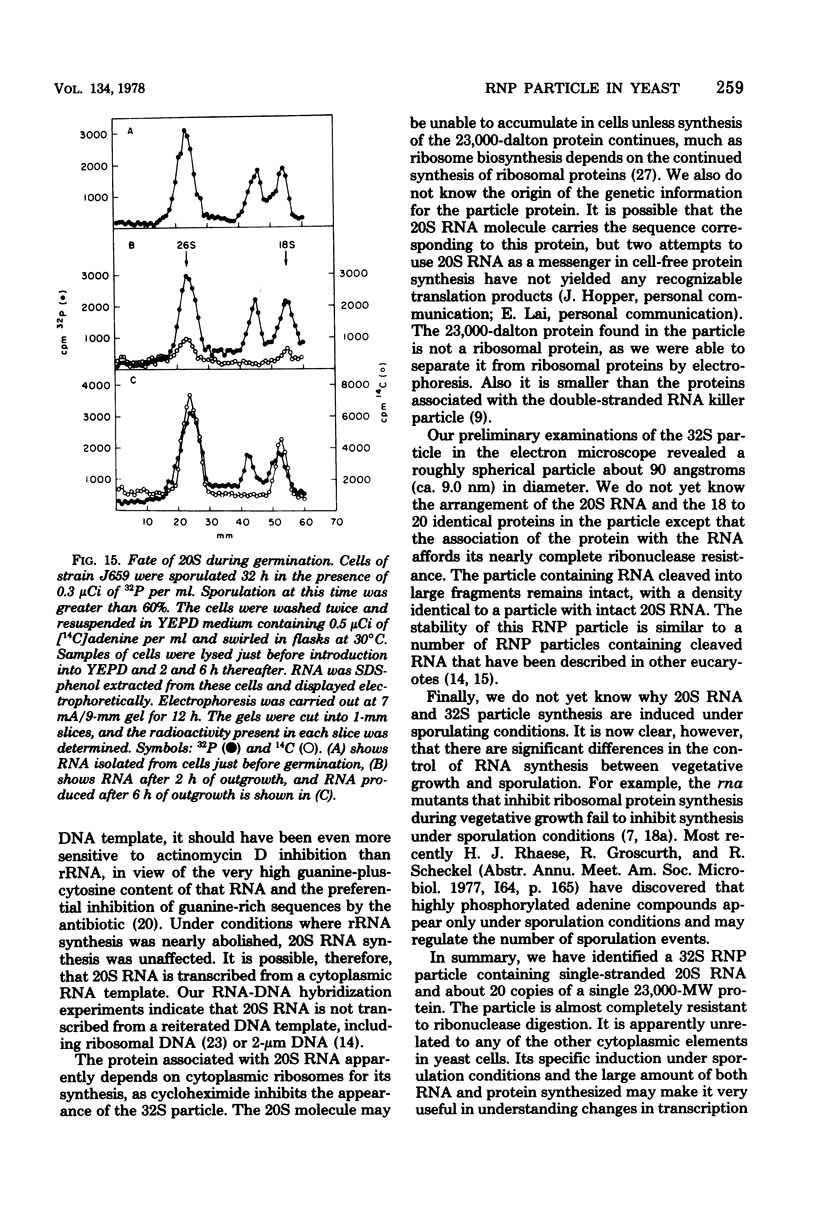
During sporulation of Saccharomyces cerevisiae, most strains accumulate an unmethylated 20S RNA. Contrary to previous reports, this sporulation 20S RNA is distinct from the short-lived methylated 20S RNA precursor of 18S rRNA. This RNA species was found in a cytoplasmic 32S ribonucleoprotein particle consisting of one single-stranded 20S RNA molecule and 18 to 20 identical protein subunits of molecular weight 23,000. The ribonucleoprotein particle was resistant to ribonuclease digestion, although purified 20S RNA was ribonuclease sensitive. Both the RNA and the protein of the 32S ribonucleoprotein particle were only synthesized under conditions that induce sporulation. The accumulation of 20S RNA depended on continued protein synthesis but was actinomycin D insensitive, despite a high guanine-plus-cytosine content. Synthesis of 20S RNA stopped when cells were removed from sporulation conditions and placed in growth medium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S. Isopycnic separation of subcellular components from poliovirus-infected and normal HeLa cells. Science. 1968 Nov 1;162(3853):572–574. doi: 10.1126/science.162.3853.572. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Miklos G. L. Localization and quantification of circular DNA in yeast. Eur J Biochem. 1974 Jan 16;41(2):359–365. doi: 10.1111/j.1432-1033.1974.tb03278.x. [DOI] [PubMed] [Google Scholar]
- Garvik B., Haber J. E. New cytoplasmic genetic element that controls 20S RNA synthesis during sporulation in yeast. J Bacteriol. 1978 Apr;134(1):261–269. doi: 10.1128/jb.134.1.261-269.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein C., Warner J. R. Coordinate regulation of the synthesis of eukaryotic ribosomal proteins. Proc Natl Acad Sci U S A. 1976 May;73(5):1547–1551. doi: 10.1073/pnas.73.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N., Phillips S. Length heterogeneity in the poly (adenylic acid) region of yeast messenger ribonucleic acid. Biochemistry. 1974 Dec 17;13(26):5378–5383. doi: 10.1021/bi00723a020. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S., Warner J. R. Identification of ten genes that control ribosome formation in yeast. Mol Gen Genet. 1970;109(1):42–56. doi: 10.1007/BF00334045. [DOI] [PubMed] [Google Scholar]
- Herring A. J., Bevan E. A. Virus-like particles associated with the double-stranded RNA species found in killer and sensitive strains of the yeast Saccharomyces cerevisiae. J Gen Virol. 1974 Mar;22(3):387–394. doi: 10.1099/0022-1317-22-3-387. [DOI] [PubMed] [Google Scholar]
- Hopper A. K., Magee P. T., Welch S. K., Friedman M., Hall B. D. Macromolecule synthesis and breakdown in relation to sporulation and meiosis in yeast. J Bacteriol. 1974 Aug;119(2):619–628. doi: 10.1128/jb.119.2.619-628.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
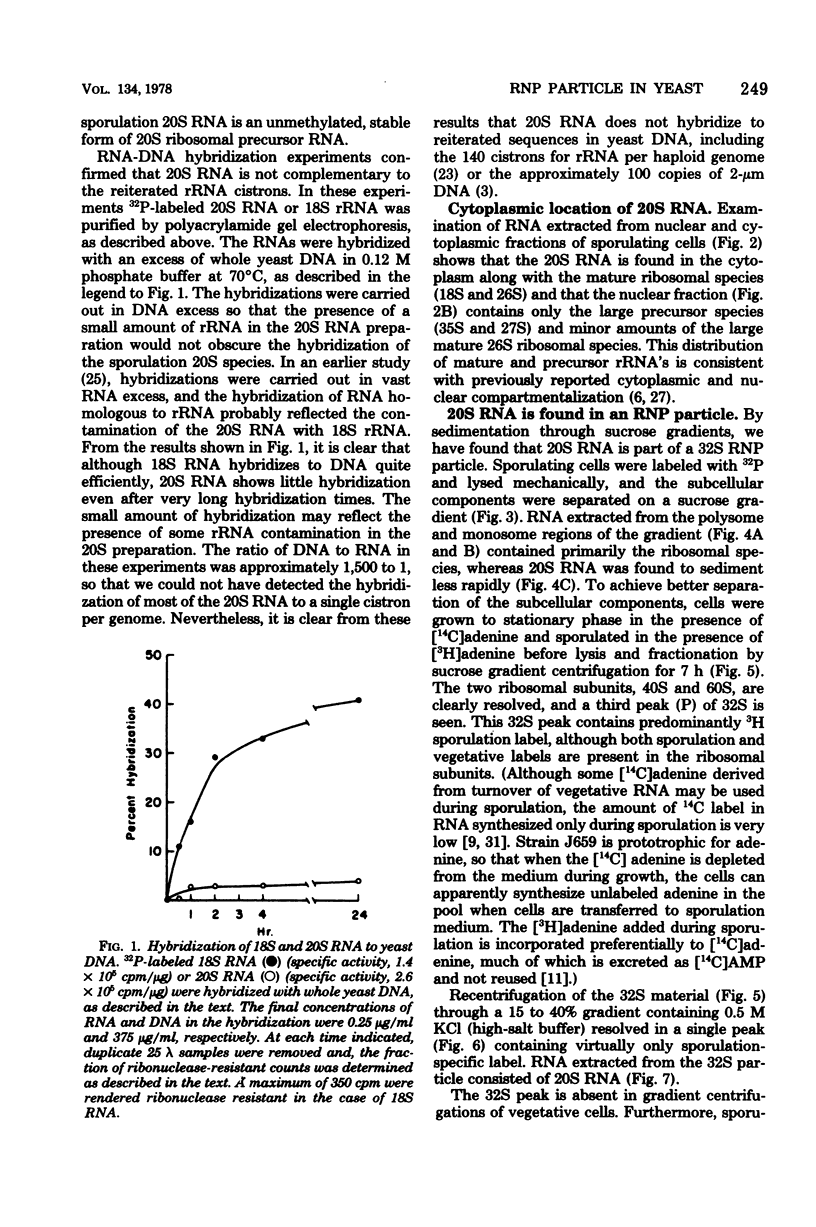
- Kadowaki K., Halvorson H. O. Appearance of a new species of ribonucleic acid during sporulation in Saccharomyces cerevisiae. J Bacteriol. 1971 Mar;105(3):826–830. doi: 10.1128/jb.105.3.826-830.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki K., Halvorson H. O. Isolation and properties of a new species of ribonucleic acid synthesized in sporulating cells of Saccharomyces cerevisiae. J Bacteriol. 1971 Mar;105(3):831–836. doi: 10.1128/jb.105.3.831-836.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
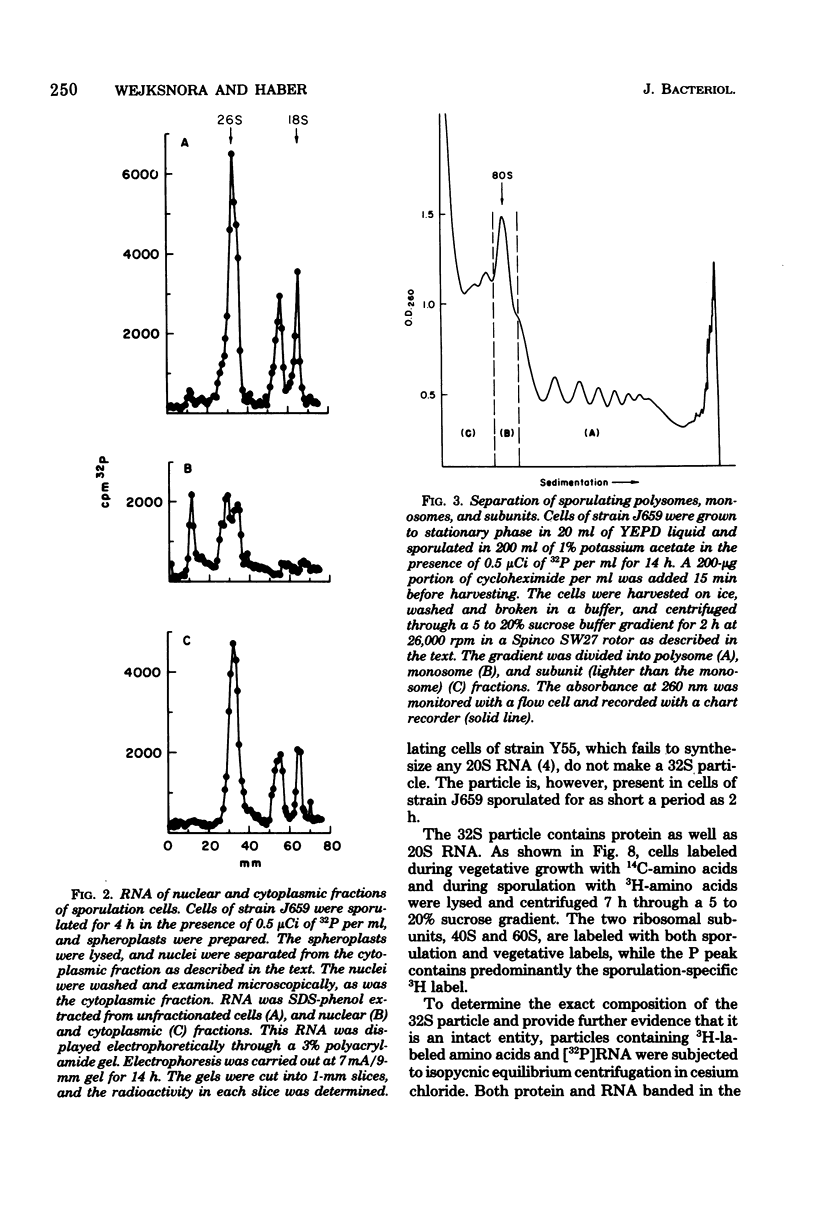
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lava-Sanchez P. A., Puppo S. Occurrence in vivo of "hidden breaks" at specific sites of 26 S ribosomal RNA of Musca carnaria. J Mol Biol. 1975 Jun 15;95(1):9–20. doi: 10.1016/0022-2836(75)90331-9. [DOI] [PubMed] [Google Scholar]
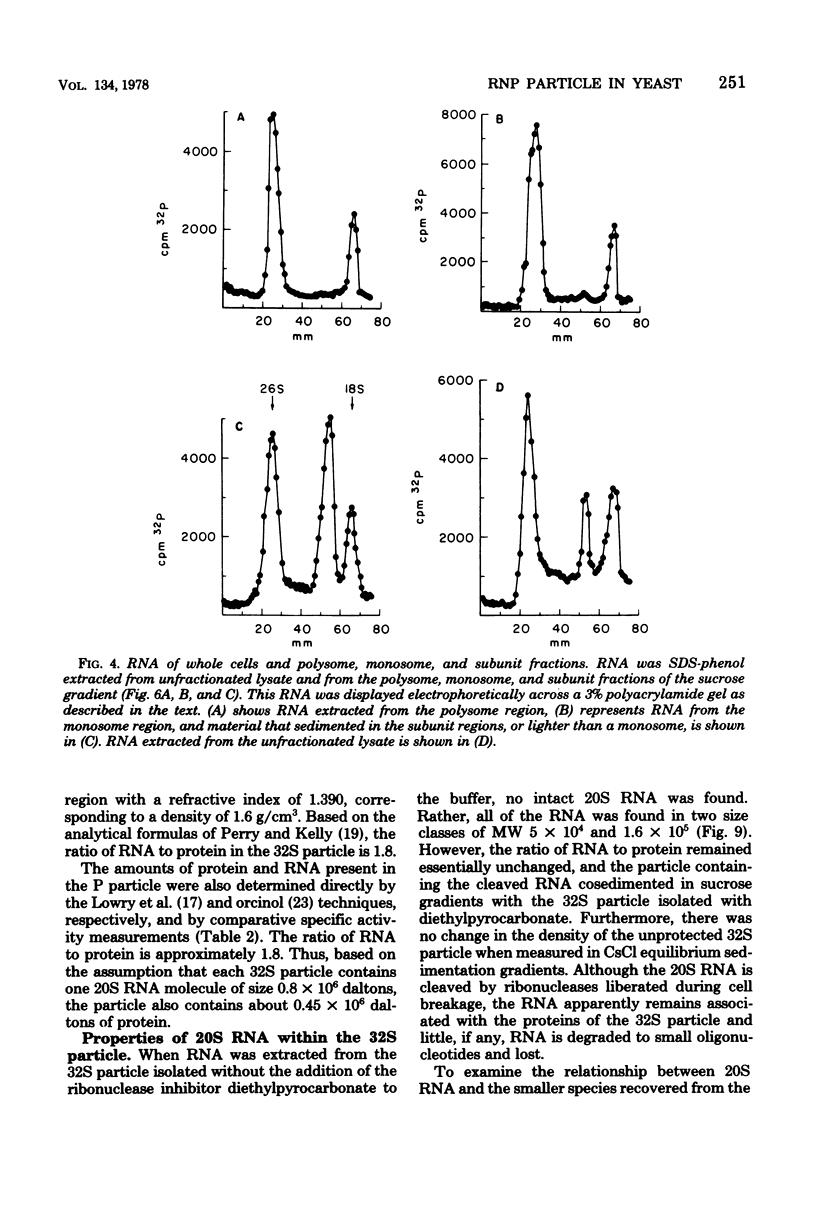
- Lemke P. A., Nash C. H. Fungal viruses. Bacteriol Rev. 1974 Mar;38(1):29–56. doi: 10.1128/br.38.1.29-56.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J. Thermal denaturation of deoxyribosenucleic acid isolated from a thermophile. Biochim Biophys Acta. 1960 Feb 26;38:342–343. doi: 10.1016/0006-3002(60)91251-8. [DOI] [PubMed] [Google Scholar]
- Pearson N. J., Haber J. E. Changes in regulation of ribosome synthesis during different stages of the life cycle of Saccharomyces cerevisiae. Mol Gen Genet. 1977 Dec 14;158(1):81–91. doi: 10.1007/BF00455122. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Buoyant densities of cytoplasmic ribonucleoprotein particles of mammalian cells: distinctive character of ribosome subunits and the rapidly labeled components. J Mol Biol. 1966 Apr;16(2):255–268. doi: 10.1016/s0022-2836(66)80171-7. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Inhibition of RNA synthesis by actinomycin D: characteristic dose-response of different RNA species. J Cell Physiol. 1970 Oct;76(2):127–139. doi: 10.1002/jcp.1040760202. [DOI] [PubMed] [Google Scholar]
- Popa L. M., Repanovici R. The study of toluidine blue-ribosomal RNA complexes. Biochim Biophys Acta. 1969 May 20;182(1):158–168. doi: 10.1016/0005-2787(69)90530-9. [DOI] [PubMed] [Google Scholar]
- Schweizer E., MacKechnie C., Halvorson H. O. The redundancy of ribosomal and transfer RNA genes in Saccharomyces cerevisiae. J Mol Biol. 1969 Mar 14;40(2):261–277. doi: 10.1016/0022-2836(69)90474-4. [DOI] [PubMed] [Google Scholar]
- Sherton C. C., Wool I. G. The extraction of proteins from eukaryotic ribosomes and ribosomal subunits. Mol Gen Genet. 1974;135(2):97–112. doi: 10.1007/BF00264778. [DOI] [PubMed] [Google Scholar]
- Sogin S. J., Haber J. E., Halvorson H. O. Relationship between sporulation-specific 20S ribonucleic acid and ribosomal ribonucleic acid processing in Saccharomyces cerevisiae. J Bacteriol. 1972 Nov;112(2):806–814. doi: 10.1128/jb.112.2.806-814.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney T. K., Tate A., Fink G. R. A study of the transmission and structure of double stranded RNAs associated with the killer phenomenon in Saccharomyces cerevisiae. Genetics. 1976 Sep;84(1):27–42. doi: 10.1093/genetics/84.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udem S. A., Warner J. R. The cytoplasmic maturation of a ribosomal precursor ribonucleic acid in yeast. J Biol Chem. 1973 Feb 25;248(4):1412–1416. [PubMed] [Google Scholar]
- Vodkin M., Katterman F., Fink G. R. Yeast killer mutants with altered double-stranded ribonucleic acid. J Bacteriol. 1974 Feb;117(2):681–686. doi: 10.1128/jb.117.2.681-686.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R. The assembly of ribosomes in yeast. J Biol Chem. 1971 Jan 25;246(2):447–454. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wejksnora P. J., Haber J. E. Methionine-dependent synthesis of ribosomal ribonucleic acid during sporulation and vegetative growth of Saccharomyces cerevisiae. J Bacteriol. 1974 Dec;120(3):1344–1355. doi: 10.1128/jb.120.3.1344-1355.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]