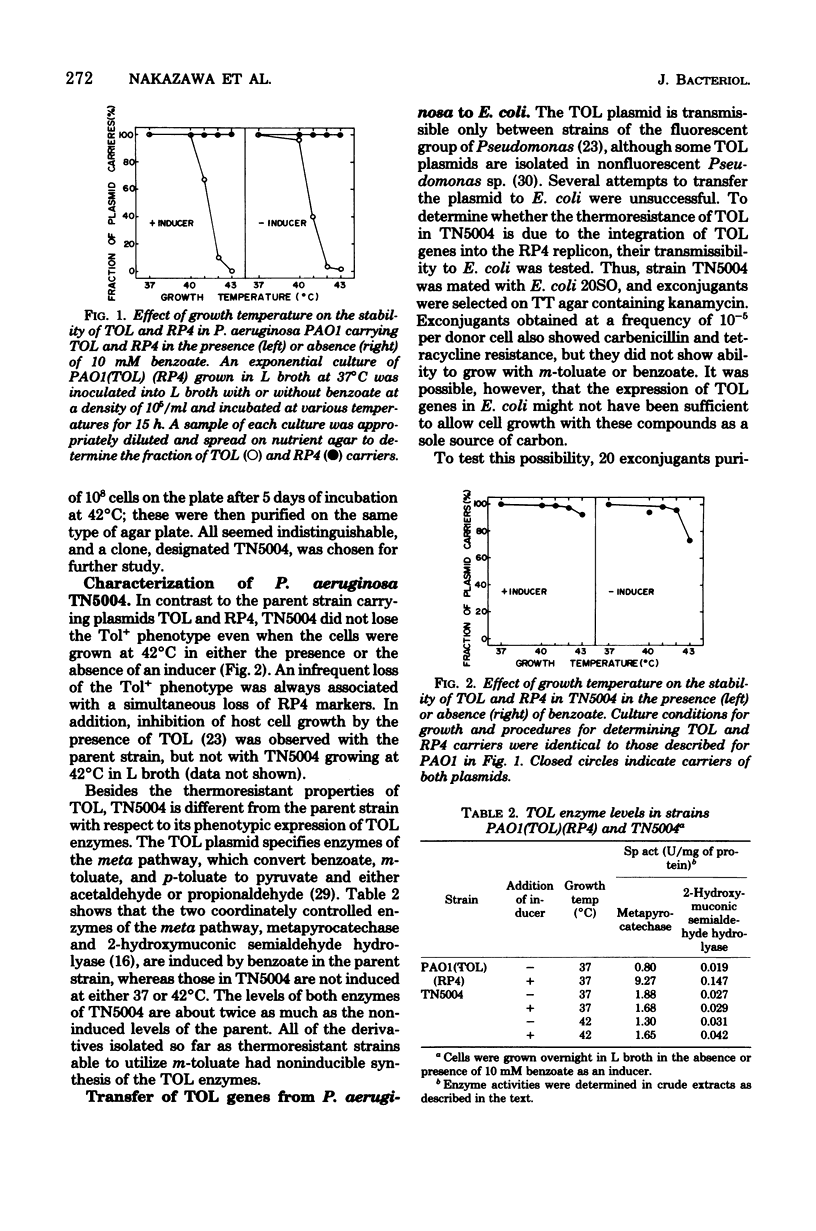
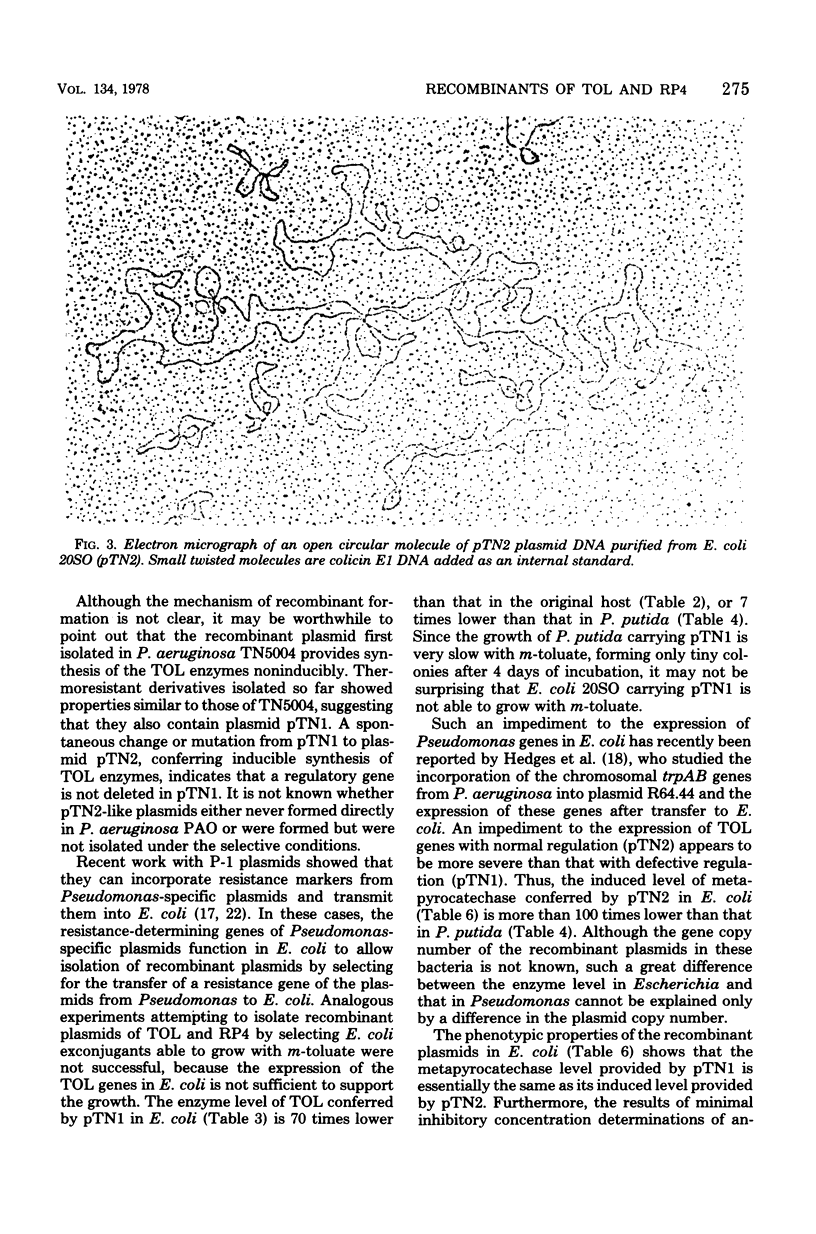
Abstract
We obtained genetic and molecular evidence of non-thermosensitive recombinants of RP4 (Kmr Tcr Cbr/Apr) and the thermosensitive TOL plasmid. As first isolated in Pseudomonas aeruginosa PAO, the recombinant plasmid pTN1 specified noninducible synthesis of TOL enzymes and was transmissible to Escherichia coli on selection for the transfer of kanamycin resistance. The phenotypic expression of TOL genes of pTN1 in E. coli was low and also noninducible. A spontaneous segregant, pTN2, appearing from pTN1, conferred inducible synthesis of TOL enzymes. These plasmids carry all of the TOL determinants as evidenced by the ability of Pseudomonas putida carrying recombinant plasmids to grow on toluene, xylene, and m-toluate. In E. coli the expression of TOL genes with normal regulation (pTN2) appears to be extremely low without induction, and the induced expression is comparable to that with defective regulation (pTN1). The measurement of the molecular weight of pTN2 by electron microscopy gave a value of about 74 X 10(6).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley S. A., Duggleby C. J., Worsey M. J., Williams P. A., Hardy K. G., Broda P. Two modes of loss of the Tol function from Pseudomonas putida mt-2. Mol Gen Genet. 1977 Jul 20;154(2):203–204. doi: 10.1007/BF00330838. [DOI] [PubMed] [Google Scholar]
- Benedik M., Fennewald M., Shapiro J. Transposition of a beta-lactamase locus from RP1 into Pseudomonas putida degradative plasmids. J Bacteriol. 1977 Feb;129(2):809–814. doi: 10.1128/jb.129.2.809-814.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Semaka S. D., Van den Elzen H. M., Kinnear J. E., Whitehouse R. L. Characteristics of R931 and other Pseudomonas aeruginosa R factors. Antimicrob Agents Chemother. 1973 May;3(5):625–637. doi: 10.1128/aac.3.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Shahrabadi M. S., van den Elzen H. M. Gentamicin resistance in Pseudomonas aeruginosa: R-factor-mediated resistance. Antimicrob Agents Chemother. 1974 Aug;6(2):191–199. doi: 10.1128/aac.6.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M. Genetic fusion of incompatible plasmids in Pseudomonas. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1641–1644. doi: 10.1073/pnas.70.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M. Plasmids in Pseudomonas. Annu Rev Genet. 1976;10:7–30. doi: 10.1146/annurev.ge.10.120176.000255. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Host ranges of R factors. J Gen Microbiol. 1972 May;70(3):453–460. doi: 10.1099/00221287-70-3-453. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby C. J., Bayley S. A., Worsey M. J., Williams P. A., Broda P. Molecular sizes and relationships of TOL plasmids in Pseudomonas. J Bacteriol. 1977 Jun;130(3):1274–1280. doi: 10.1128/jb.130.3.1274-1280.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesberg E., Wilcox G. Regulation: positive control. Annu Rev Genet. 1974;8:219–242. doi: 10.1146/annurev.ge.08.120174.001251. [DOI] [PubMed] [Google Scholar]
- Falkow S., Guerry P., Hedges R. W., Datta N. Polynucleotide sequence relationships among plasmids of the I compatibility complex. J Gen Microbiol. 1974 Nov;85(1):65–76. doi: 10.1099/00221287-85-1-65. [DOI] [PubMed] [Google Scholar]
- Feist C. F., Hegeman G. D. Phenol and benzoate metabolism by Pseudomonas putida: regulation of tangential pathways. J Bacteriol. 1969 Nov;100(2):869–877. doi: 10.1128/jb.100.2.869-877.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Jacob A. E., Crawford I. P. Wide ranging plasmid bearing the Pseudomonas aeruginosa tryptophan synthase genes. Nature. 1977 May 19;267(5608):283–284. doi: 10.1038/267283a0. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Jacob A. E. Mobilization of plasmid-borne drug resistance determinants for transfer from Pseudomonas aeruginosa to Escherichia coli. Mol Gen Genet. 1975 Sep 15;140(1):69–79. doi: 10.1007/BF00268990. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T., Yura T., Yamagishi H. Genetic and physical studies of lambda transducing bacteriophage carrying the beta subunit gene of the Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1975 Jun;122(3):1247–1256. doi: 10.1128/jb.122.3.1247-1256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Jacob A. E., Hedges R. W. Recombination between plasmids of incompatibility groups P-1 and P-2. J Bacteriol. 1976 Sep;127(3):1278–1285. doi: 10.1128/jb.127.3.1278-1285.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T. TOL plasmid in Pseudomonas aeruginosa PAO: thermosensitivity of self-maintenance and inhibition of host cell growth. J Bacteriol. 1978 Feb;133(2):527–535. doi: 10.1128/jb.133.2.527-535.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T., Yokota T. Benzoate metabolism in Pseudomonas putida(arvilla) mt-2: demonstration of two benzoate pathways. J Bacteriol. 1973 Jul;115(1):262–267. doi: 10.1128/jb.115.1.262-267.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H., Shipley P. Host range and properties of the Pseudomonas aeruginosa R factor R1822. J Bacteriol. 1973 Feb;113(2):772–780. doi: 10.1128/jb.113.2.772-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton J. M., Holloway B. W. Chromosome mapping in Pseudomonas aeruginosa. Genet Res. 1972 Jun;19(3):251–260. doi: 10.1017/s0016672300014518. [DOI] [PubMed] [Google Scholar]
- Williams P. A., Murray K. Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J Bacteriol. 1974 Oct;120(1):416–423. doi: 10.1128/jb.120.1.416-423.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. A., Worsey M. J. Ubiquity of plasmids in coding for toluene and xylene metabolism in soil bacteria: evidence for the existence of new TOL plasmids. J Bacteriol. 1976 Mar;125(3):818–828. doi: 10.1128/jb.125.3.818-828.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsey M. J., Williams P. A. Metabolism of toluene and xylenes by Pseudomonas (putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975 Oct;124(1):7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Yokota T. Host-dependent, thermosensitive replication of an R plasmid, pJY5, isolated from Enterobacter cloacae. J Bacteriol. 1977 Dec;132(3):923–930. doi: 10.1128/jb.132.3.923-930.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]




