Abstract
Light-dependent activation of thylakoid protein phosphorylation regulates the energy distribution between photosystems I and II of oxygen-evolving photosynthetic eukaryotes as well as the turnover of photosystem II proteins. So far the only known effect of light on the phosphorylation process is the redox-dependent regulation of the membrane-bound protein kinase(s) activity via plastoquinol bound to the cytochrome bf complex and the redox state of thylakoid dithiols. By using a partially purified thylakoid protein kinase and isolated native chlorophyll (chl) a/b light-harvesting complex II (LHCII), as well as recombinant LHCII, we find that illumination of the chl-protein substrate exposes the phosphorylation site to the kinase. Light does not activate the phosphorylation of the LHCII apoprotein nor the recombinant pigment-reconstituted complex lacking the N-terminal domain that contains the phosphothreonine site. The suggested light-induced conformational change exposing the N-terminal domain of LHCII to the kinase is evidenced also by an increase in its accessibility to tryptic cleavage after light exposure. Light activates preferentially the trimeric form of LHCII, and the process is paralleled by chl fluorescence quenching. Both phenomena are slowly reversible in darkness. Light-induced exposure of the LHCII N-terminal domain to the endogenous protein kinase(s) and tryptic cleavage occurs also in thylakoid membranes. These results demonstrate that light may regulate thylakoid protein phosphorylation not only via the signal transduction chain connecting redox reactions to the protein kinase activation, but also by affecting the conformation of the chl-protein substrate.
Redox-controlled thylakoid protein phosphorylation in photosynthetic eukaryotes plays an important role in the regulation of the light energy distribution between the two photosystems (PSs) and controls the light-induced turnover of PSII reaction center subunits. The reversible association/dissociation of a mobile pool of the light-harvesting chlorophyll (chl) a/b protein complex (LHCII) with PSII (state I to state II transition) is ascribed to the redox-controlled activation/deactivation of thylakoid-bound protein kinase(s) and phosphorylation of the LHCII proteins (1, 2). Phospho-LHCII dissociates from PSII and transfers energy to PSI (state II, reviewed in ref. 2). Phospho-LHCII is dephosphorylated by a thylakoid-bound phosphatase (3–5) regulated by a recently discovered cyclophilin-like protein, TLP40, localized in the thylakoid lumen (6, 7). After dephosphorylation, LHCII reassociates with PSII (state I, refs. 7–9). The state transition process involves lateral migration of LHCII (10) and cytochrome bf complex from the appressed to the nonappressed thylakoid domains, thereby enhancing PSI cyclic electron flow (11).
The signal transduction loop interconnecting light-driven electron flow with the thylakoid protein kinase(s) activation involves the interaction of reduced plastoquinone with the quinol oxidation site of the cytochrome bf complex whose electron carriers of the high potential path, the Rieske Fe-S center and cytochrome f, are reduced (7, 12–15). So far, the role of light during thylakoid protein phosphorylation was ascribed solely to redox activation of the protein kinase(s). Recently, it also was reported that the redox state of thylakoid thiols plays a regulatory role in the thylakoid protein phosphorylation (16). By using a partially purified protein kinase preparation obtained from spinach thylakoids and isolated native or recombinant LHCII, we demonstrate that light also may affect the accessibility of the phosphorylation site of LHCII to the kinase in an in vitro reconstituted system. Illumination also enhances the accessibility of LHCII to the endogenous thylakoid membrane kinase.
We propose that light plays a dual role in the process of regulation of the thylakoid protein phosphorylation, that of enzyme activation via the redox signal transduction system as well as enhancing the exposure of the chl-protein substrate phosphorylation site to the protein kinase.
MATERIALS AND METHODS
Preparation of Kinase Active Fractions.
Spinach thylakoids prepared as in ref. 17 were suspended in 10 mM Tris⋅HCl (pH 8.0), 0.4 M sucrose, and 10 mM NaCl and stored at −70°C. Crude protein extracts were prepared from the thylakoids and a protein-kinase enriched fraction (AMS) was obtained by ammonium sulfate precipitation (35–55% saturation) according to ref. 18.
The AMS precipitate was dissolved in 25 mM Tris⋅HCl (pH 7.5) containing 25 mM β-d-octyl glucoside, 200 μM phenazine methosulfate, and 1 mM benzamidine. Further fractionation after treatment of AMS with 1.0 M LiClO4 (pH 6.0) in the cold for 10 min and desalting on Sephadex G-25 columns was performed by ion-exchange perfusion chromatography (POROS-Q, PerSeptive Biosystems, Framingham, MA) (20, 21) using an HPLC apparatus (Merck-Hitachi, L-6200A). Fractions (1 ml) were eluted at 4°C by a NaCl gradient (0–0.7 M) containing 5 mM 3-[(chloroamidopropyl)dimethyl-ammonio]-1-propansulfonate and 0.1% Triton X-100. The peak of kinase activity was found typically in fractions 17–22. Kinase fractions exhibiting up to 800-fold enrichment of activity on a protein basis (19) were stored at −70°C until use. Kinase activity of protein bands in the various fractions was detected by electro-transfer of the proteins after denaturing SDS/PAGE to poly(vinylidene difluoride) membranes followed by renaturation of the kinase as in ref. 22. The renatured kinase bands did not exhibit self-phosphorylation and were detected by using 32P-γ-ATP-Mg and histone S-III as a substrate. Cytochrome f, the Rieske protein, and polyphenol-oxidaze were detected by immunodecoration (23) using monospecific polyclonal antibodies.
Phosphorylation Assay.
The phosphorylation reaction mixture (100 μl) contained 50 mM Tris⋅HCl (pH, 8.0), 10 mM NaCl, 10 mM MgCl2, 0.2 mM ATP (25 μC 32P-γ-ATP mmol−1), 10 mM NaF, 0.5 mM 3-[(chloroamidopropyl)dimethyl-ammonio]-1-propansulfonate, 0.01% Triton X-100, chl-protein substrate equivalent to 2.5 μg chl or 5 μg of pigment-free protein, and finally 5 μg protein of the kinase fraction. Unless otherwise specified, all incubations were carried out in Eppendorf tubes at 25°C in dim light (<5 μmol m−2⋅s−1) or in darkness. Phosphorylation was terminated by addition of SDS/PAGE sample buffer, and aliquots were counted for 32P-phosphoprotein content (19). Additional aliquots were taken for resolution of the phosphorylated polypeptides by denaturing SDS/PAGE (24) and autoradiography. The radioactive labeling of LHCII was measured by scanning autoradiograms exposed for linear response. Labeling of LHCII monomers and trimers was determined by scanning of the chl-proteins resolved by nondenaturing gel electrophoresis (25). Detection of nonradioactively labeled phosphoproteins was carried out by immunodecoration using antiphospho-threonine antibodies (26).
Preparation of Phosphorylation Substrates.
Native LHCII was prepared from 9- to 11-day-old pea plants according to ref. 27 and stored at −70°C in 0.5% nonyl-β-d-glucoside. Recombinant LHCII was obtained from bacterial overexpressed protein, either as a wild-type protein with the authentic amino acid sequence of Lhcb1*2 in pea or as an N-terminally truncated version lacking 11 aa (clone ΔN-11 in 28). Reconstitution of the complexes with added chl and carotenoids was as described (28). Histone S-III was purchased from Sigma.
Light Exposure, Trypsin Treatment, and Fluorescence Measurements.
Isolated and recombinant LHCII substrates were suspended in 100-μl reaction mixture and exposed to white light (200 μmol photons m−2⋅s−1) at 25°C in the presence of the kinase and ATP-Mg. For preillumination experiments, LHCII suspended in the phosphorylation reaction mixture lacking the enzyme was exposed to light as above before the addition of the kinase and further incubation in darkness.
Light-induced exposure of LHCII to phosphorylation in situ was detected by preincubation of thylakoids in the light (50 μmol photons m−2⋅s−1) or in darkness for 5 min while preventing the thylakoid endogenous kinase activation by addition of 10 μM 3-(3,4-dichlorophenyl)-1,1-dimethyl-urea (DCMU) during the illumination. Phosphorylation was allowed to occur in darkness for 20 min in the presence of 32P-γ-ATP-Mg without or with addition of 1 mM duroquinol to activate the kinase. As a control, thylakoids also were phosphorylated in light in the presence or absence of DCMU without the addition of duroquinol.
To test the effect of preillumination on the tryptic digestion of LHCII, isolated native LHCII samples equivalent to 25 μg of chl were preincubated for 10 min in 100 μl of the phosphorylation buffer in the light (200 μmol photons m−2⋅s−1) or in darkness. The samples then were incubated at room temperature in darkness for 2 min with the addition of trypsin (0.2 μg).
For tryptic digestion of LHCII in situ thylakoids equivalent to 25 μg of chl suspended in a final volume of 100 μl of 50 mM Tris⋅HCl buffer, pH 7.5, containing 5 mM MgCl2 and 10 mM NaCl were incubated for 5 min in the light (30 μmol photons m−2⋅s−1) with addition of 10 μM DCMU to prevent the kinase activation or in darkness. Trypsin (0.2 μg ml−1) was added, and incubation was continued in darkness for 10 min at 25°C. Trypsin was obtained from Sigma (type XIII, bovine pancreas), and proteolysis was terminated by the addition of soybean trypsin inhibitor (Sigma) at a ratio of 4:1 inhibitor/trypsin (wt/wt).
Fluorescence of isolated LHCII (1 ml, 1.6–1.8 OD at 430 nm) elicited at 22°C by actinic light of various intensities was measured by using a pulse amplitude-modulated fluorimeter (Waltz, Effeltrich, Germany) and the fluorescence emission was monitored by the modulated beam (650 nm, 1.6 kHz, light intensity 0.01 μmol m−2⋅s−1). Fluorescence emission and excitation spectra at 77 K were recorded as described (29).
RESULTS
Phosphorylation of LHCII by Partially Purified Protein Kinase.
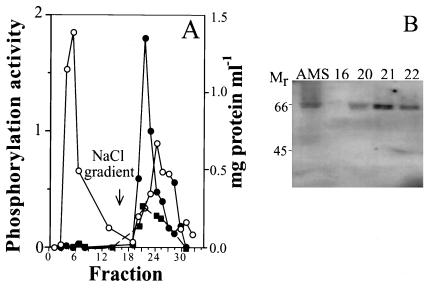
The phosphorylation activity of fractions obtained after the purification of a thylakoid protein extract by perfusion chromatography, using histone S-III and isolated LHCII as substrates, is shown in Fig. 1A. Resolution of the protein pattern of the partially purified kinase fraction by SDS/PAGE disclosed the presence of about 10 polypeptide bands. Among these, cytochrome f, the Rieske protein, and polyphenol-oxidaze were identified by immunoblotting (not shown). A correlation was found between the protein kinase activity and the presence of a major polypeptide band, possibly a doublet, at about 65 kDa detected by its kinase activity after denaturing SDS/PAGE and renaturation on poly(vinylidene difluoride) membrane. The 65-kDa band was present in the AMS and the kinase-enriched chromatography fractions 20–22 but not in the nonactive fraction 16 (Fig. 1B).
Figure 1.
Thylakoid protein kinase purification by perfusion chromatography. (A) Protein elution and activity profile of protein kinase fractionation by perfusion chromatography; ○, protein concentration; ● and ■, kinase activity measured with histone S-III and isolated LHCII as substrates, respectively. (B) Presence of a protein kinase band(s) at about 65 kDa in the ammonium sulfate precipitated fraction from the crude thylakoid extract (AMS) and in the peak active fractions 20–22 of A detected by renaturation of kinase activity of SDS/PAGE resolved proteins as described in Materials and Methods; phosphorylation activity is given in nmoles 32P-mg protein−1.
Effect of Illumination on the Phosphorylation of Native and Recombinant LHCII.
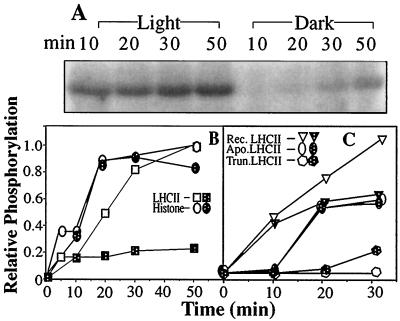
Illumination of the phosphorylation assay increases several-fold the labeling of native isolated LHCII (Fig. 2 A and B) but not that of histone S-III as compared with a dark control (Fig. 2B). To determine whether light affects specifically the phosphorylation of the chl-protein LHCII complex, recombinant LHCII apoprotein was used as a substrate before or after reconstitution with pigments (28). The recombinant apoprotein is a relatively poor substrate and illumination did not increase its phosphorylation. The phosphorylation in darkness of recombinant LHCII reconstituted with pigments was only slightly higher than that of the apoprotein and increased in the light by about 40% (Fig. 2C). Because in the reconstitution system only about 20% of the apoprotein is associated with pigments (28) these results indicate that light indeed affects the recombinant LHCII chl-protein.
Figure 2.
Kinetics of light activation of native and recombinant LHCII phosphorylation by the partially purified protein kinase. (A) Autoradiogram of isolated native LHCII phosphorylation in the light and darkness. (B) Quantification (average of three experiments ± 10%) of autoradiograms from similar experiments using LHCII or histone-S-III as substrates. (C) Phosphorylation of recombinant LHCII. The radioactivity incorporated in the light-incubated phosphorylation assay (nmol 32P-mg protein−1 at the end of the incubation) is taken as the maximal value for each substrate [4.6 and 7.5 for native LHCII and histone and 3.0 and 1.5 for the recombinant LHCII reconstituted with pigments (Rec. LHCII) and the apoprotein, (Apo. LHCII), respectively]; the values for the recombinant N-terminal truncated LHCII (Trun. LHCII) reconstituted with pigments are within the background noise level of the assay (about 0.1 nmol 32P-mg protein−1); open and dotted symbols, light- and dark-incubated samples, respectively.
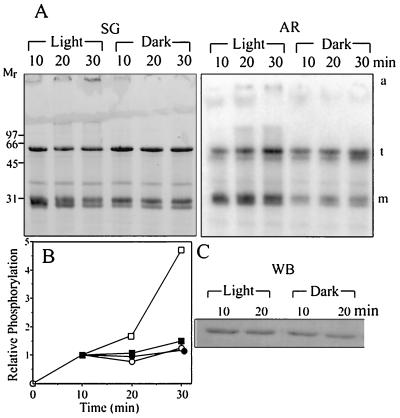
LHCII associated with PSII in situ assumes a trimeric form (reviewed in refs. 2 and 30). Thus, it was of interest to determine whether light specifically activates the phosphorylation of the oligomeric form of the LHCII substrate. Isolated pea LHCII consisting of a mixture of monomers and trimers therefore was phosphorylated in the light or darkness. Subsequently monomers and trimers were resolved by nondenaturing gel electrophoresis, and their radioactive labeling was determined by autoradiography. As shown in Fig. 3, illumination increases the phosphorylation of both the monomer and trimer LHCII relative to the dark control. However, light affects the phosphorylation of the monomers only during the first 10 min of incubation, whereas the effect of light on the phosphoryaltion of LHCII trimers continues for up to 30 min (Fig. 3A). The phosphorylation of LHCII, measured by scanning the autoradiogram and relating the intensity to the protein content by scanning the stained gels, indicates that light preferentially activates the phosphorylation of the LHCII trimers (Fig. 3B). Small amounts of LHCII that did not penetrate the running gel are formed with increasing illumination and could represent aggregates of denatured LHCII. These aggregates are not significantly phosphorylated as compared with the LHCII monomers and trimers (Fig. 3A).
Figure 3.
Effect of illumination on the phosphorylation of LHCII monomers and trimers. Isolated LHCII was phosphorylated by the solubilized kinase in the light or darkness. (A) The stained gel (SG) of the phosphorylation mixture resolved by nondenaturing SDS/PAGE into monomers (m) and trimers (t). AR, autoradiogram. (B) Quantification of the autoradiogram in A. The phosphorylation level of all samples at 10-min incubation is taken as one unit. (C) The phosphorylation mixture was subjected to denaturing SDS/PAGE that does not separate LHCII monomers from trimers, and the phosphorylation of the total LHCII band was detected after transfer to poly(vinylidene difluoride) membrane by phosphothreonine antibodies; squares and circles, trimer and monomer forms of LHCII phosphorylated in the light (open symbols) or darkness (closed symbols), respectively. a, large molecular mass aggregates; WB, Western blot.
Illumination of the in vitro phosphorylation assay increased only slightly the phosphothreonine content of total LHCII relative to the sample phosphorylated in darkness as detected by phosphothreonine antibodies after denaturing SDS/PAGE (Fig. 3C). This finding could be explained if one considers that part of the phosphothreonine sites already is esterified in vivo before the LHCII isolation as detected by this method (not shown).
Effect of Illumination on the Exposure of the LHCII N-Terminal Phosphorylation Site.
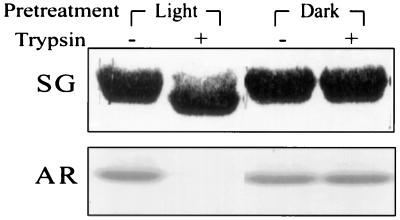
The threonine residue that is the phosphorylation site of LHCII (2) is located on its N-terminal hydrophilic domain exposed in situ at the outer surface of the appressed grana thylakoid membranes. The N-terminal deleted recombinant LHCII apoprotein reconstituted with pigments and lacking the phosphothreonine site was not phosphorylated by the added kinase (Fig. 2C). This domain of LHCII is also highly sensitive to trypsin, which cleaves a segment of about 2 kDa containing the phosphothreonine site (31). To determine whether a putative light-induced conformational change affects this N-terminal region, enhancing its exposure to the protein kinase, we have tested the effect of light on the accessibility of the isolated LHCII N-terminal domain to tryptic cleavage. The results demonstrate that preillumination of native LHCII facilitates the subsequent cleavage of the N-terminal domain by trypsin in darkness as indicated by the typical increase in the electrophoretic mobility of the residual LHCII (Fig. 4). Notably, a concomitant loss of the phosphorylation site occurs as indicated by the lack of radioactive labeling of the substrate.
Figure 4.
Effect of illumination on the tryptic cleavage of the LHCII N-terminal domain and removal of the phosphorylation site. LHCII suspended in the phosphorylation buffer was incubated in the light or darkness for 20 min. The samples were transferred to darkness, trypsin was added, and proteolysis was stopped after 2 min followed by addition of the kinase and phosphorylation in darkness for 30 min. SG, stained gel; AR, autoradiogram.
Light-Induced Activation of LHCII in the Absence of the Kinase.
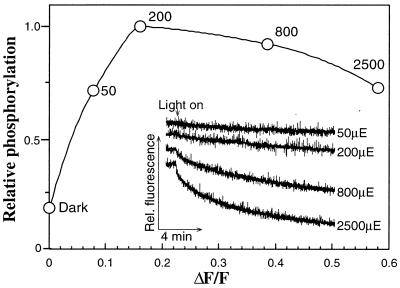
The question arises whether illumination may alter the conformation of isolated LHCII in the absence of the enzyme. To test this possibility, we have preilluminated LHCII and performed the phosphorylation by adding the kinase in darkness subsequent to the illumination (Fig. 5). LHCII phosphorylation increases with the raise in the light intensity during the preillumination and reached a plateau at about 200 μmol photons m−2⋅s−1. This saturating light level, which is in the lower range of natural daylight, does not cause bleaching of the isolated LHCII (not shown).
Figure 5.
Effect of preillumination on LHCII fluorescence quenching and subsequent phosphorylation in darkness. LHCII suspended in the phosphorylation buffer was exposed for 15 min to light of various intensities. The samples were transferred to darkness, and the partially purified kinase was added and phosphorylation was allowed to proceed in darkness for 20 min. (Inset) Kinetics of fluorescence quenching as a function of light intensity. ○, phosphorylation level of the LHCII after exposure to light intensities as indicated by the numbers (representing μmol photons m−2⋅s−1 and indicated as μE). ΔF/F, ratio of the loss of samples fluorescence after 15 min of light incubation to that of the dark control, indicating the degree of fluorescence quenching at various light intensities. The base line of the fluorescence traces in the insert is down-shifted to facilitate their visualization.
The illumination of LHCII that leads to an increase in its phosphorylation level also induces quenching of the LHCII fluorescence (Fig. 5). The fluorescence emission at 77 K of control LHCII shows a maximum at 681 nm and a low shoulder at 695 nm. After illumination an increase in the 695-nm emission relative to the emission of the 681 nm is observed, indicating some aggregation of LHCII. However, the excitation spectrum of the chl a emission bands (681 and 695 nm) indicates that energy transfer from chl b (absorption band at 650 nm) is not altered after illumination of LHCII (not shown). The quenching of LHCII fluorescence depends on the illumination intensity and time of exposure and varies to some extent for different preparations. Exposure of LHCII to higher light intensities known to cause photoinactivation of PSII and reversible inactivation of LHCII phosphorylation in vivo (2,500 μmol m−2⋅s−1) (32) leads to a further increase of the fluorescence quenching (Fig. 5). However, under these conditions the LHCII phosphorylation decreases slightly and some chl bleaching (less than 5%) occurs as well (not shown).
Reversibility of the Light-Induced Exposure of the LHCII N-Terminal Domain to Phosphorylation.
To test the reversibility of the light-induced activation of LHCII as a phosphorylation substrate as well as that of fluorescence quenching, both parameters were measured in (a) nonilluminated LHCII; (b) after exposure of LHCII to the light; (c) after subsequent incubation in darkness of the preilluminated LHCII; and (d) after re-exposure of the system treated as in c to the light. A relatively low light intensity was used in this experiment (220 μmol m−2⋅s−1). The results of such an experiment (Fig. 6) demonstrate that the light-induced increase in LHCII phosphorylation and fluorescence quenching are both reversible in darkness. Furthermore, the cycle of light-dark responses can be repeated without significant loss in the light-induced activation of LHCII phosphorylation.
Figure 6.
Reversibility of the light-induced activation of LHCII phosphorylation and fluorescence quenching. LHCII suspended in the phosphorylation buffer in darkness was transferred to the light (Dark > 220 μE) and incubated for 15 min, then transferred and incubated in darkness (>Dark) for 45 min to allow decay of the light-activated state and then re-exposed to the light (>220 μE) for 15 min. Samples were taken at each step, the kinase was added to each sample, and phosphorylation was allowed to proceed for 20 min in darkness. The fluorescence emission of the sample was measured at the end of each incubation period in the light or darkness, and the value of a sample kept in darkness as is taken as 100%; open and dashed bars, levels of LHCII fluorescence and phosphorylation, respectively; μE, μmol photons m−2⋅s−1.
Effect of Illumination on the LHCII Phosphorylation in Situ.
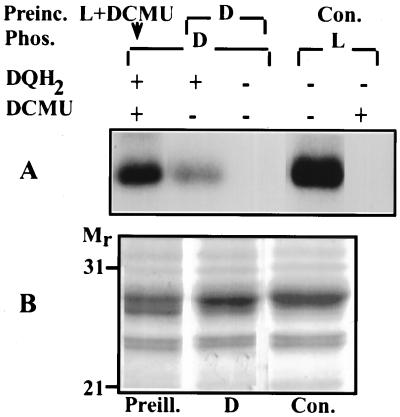
The question arises whether the illumination of LHCII exposes its N-terminal domain to the protein kinase in intact thylakoid membranes. To test the possibility that light may induce exposure of LHCII to the protein kinase in situ, isolated thylakoids were preilluminated in presence of 10 μM DCMU that completely prevented the activation of the endogenous kinase during the light exposure. The thylakoid kinase subsequently was activated in darkness by addition of duroquinol. A significant increase was obtained in the phosphorylation of LHCII in the preilluminated thylakoids during the phosphorylation in darkness as compared with nonpreilluminated control thylakoids (Fig. 7A). Preillumination of thylakoids in the presence of DCMU also enhanced exposure of the LHCII N-terminal domain to trypsin cleavage as compared with a nonpreilluminated sample (Fig. 7B). These results indicate that illumination affects the conformation of LHCII exposing the N-terminal domain to the protein kinase and trypsin in situ.
Figure 7.
Effect of illumination on the exposure of LHCII in situ (A) to the endogenous thylakoid protein kinase and (B) to cleavage of its N-terminal domain by trypsin. (A) Autoradiogram. Thylakoids preincubated (Preinc.) in the light with addition of DCMU (L + DCMU) or darkness (D) for 5 min were phosphorylated (Phos.) in darkness (D) for 20 min in the presence (+) or absence (−) of duroquinol (DQH2). As a control (Con.), thylakoids were phosphorylated in the light (L) in the absence (−) or presence (+) of DCMU. (B) Stained gel. Thylakoids preilluminated in the presence of DCMU for 5 min (Preill.) or incubated in darkness (D) were exposed to trypsin in darkness for 10 min. Con., control thylakoids not exposed to trypsin cleavage.
DISCUSSION
As part of a project aimed at the identification of the thylakoid-bound protein kinase(s) (19, 22), we have partially purified a solubilized protein kinase preparation and assayed the kinase-substrate interaction in an in vitro reconstituted system. Experimental results obtained with this system disclosed that light may contribute to the regulation of LHCII phosphorylation by affecting the conformation of its N-terminal domain.
Effect of Light on Isolated Native or Recombinant LHCII.
The results presented here show that light affects the native or recombinant LHCII reconstituted with pigments but not the recombinant apoprotein. The suggested light-induced conformational changes of LHCII involve its hydrophilic N-terminal domain containing the phosphothreonine residue(s) exposed to the stromal thylakoid membrane surface (33). This conclusion is based on the experiments showing loss of light activation of the N-terminal-deleted recombinant LHCII as well as increased tryptic cleavage of native LHCII (Figs. 2 and 4). One should note that both the native and recombinant pea LHCII used as substrates harbor several threonine residues besides the specific phosphorylation sites Thr-6 and/or Thr-7 of the N-terminal domain. Among these, Thr-38, Thr-49, and Thr-58, which are not removed by the tryptic proteolysis, are located in the hydrophilic stroma-exposed N-terminal domain, whereas Thr-227 is located in the hydrophilic C-terminal domain exposed on the luminal side of the membrane (34). These sites are not phosphorylated in thylakoids by the endogenous protein kinase as demonstrated by loss of all phosphorylated sites after trypsin cleavage of the LHCII N-terminal domain in isolated thylakoid membranes (33). The solubilized protein kinase used in this work maintains this specificity toward the phosphorylation site(s) at the N- terminal domain of both native and recombinant LHCII. Furthermore, the light-induced exposure of this site(s) to the kinase also indicates that both substrates maintain the natural conformation of the chl-protein complex exhibited by LHCII in situ.
Previous work has demonstrated that illumination of LHCII induces fluorescence quenching (35) ascribed to micro-aggregation and affects the chirality of the pigment-protein macro-complexes as indicated by CD spectroscopy (36). The aggregation and fluorescence quenching phenomena may represent parallel events induced by light but not necessarily related to the exposure of the N-terminal domain of the complex to the kinase and trypsin activity. The activation of LHCII as a kinase substrate reaches a plateau at relatively low light intensity whereas the LHCII fluorescence quenching continues to increase with further increasing light intensity. Preliminary experiments indicate that preillumination of LHCII in the absence of MgCl2 or at higher detergent concentrations, thereby minimizing macro-aggregation, does not lower the light-induced increase in LHCII phosphorylation. Furthermore, the light effect on the phosphorylation is not the result of irreversible thermal denaturation caused by heating of the LHCII in the dispersed or aggregated form. Preillumination of LHCII at 7°C or 15°C did not affect the light-induced increase in phosphorylation as compared with illumination at 22–25°C (not shown). Furthermore, the light-induced substrate activation reported here is a reversible process (Fig. 6). It is, however, possible that transient temperature jumps after excitation of the LHCII complex may contribute to the phenomena reported in this work.
Light-Induced Exposure of LHCII to the Endogenous Protein Kinase in Situ.
The light-enhanced exposure of LHCII N-terminal domain is not restricted to the in vitro reconstituted system and represents a property of LHCII in situ. Preillumination of thylakoids substantially increases the phosphorylation of LHCII in darkness as compared with a dark control. This result is not due to the activation of the thylakoid kinase during illumination because electron flow activity of PSII was inhibited during the illumination by DCMU and the kinase was activated only in darkness by addition of duroquinol.
The hydrophilic LHCII N-terminal domain is exposed on the membrane surface and may assume different conformations. Lateral aggregation leading to formation of LHCII sheets, or association of LHCII-PSII complexes, may affect its spatial orientation. Reversible light-induced structural reorganization of LHCII macro-domains ascribed to transient thermal fluctuations and ions movement has been reported to occur in thylakoid membranes as well as in isolated LHCII (37, 38). Possibly, under such conditions structural changes may occur within LHCII that may expose the N-terminal domain to the kinase.
The mechanism of the phosphorylation-induced dissociation of LHCII from PSII during the state transition process is still not fully understood. Charge repulsion between the phosphorylated LHCII and PSII cores (39) and/or conformational changes induced by the phosphorylation of the N-terminal domain of LHCII (40) may be involved in this process as indicated by NMR spectroscopy (ref. 8 and reviewed in ref. 2).
Trimeric LHCII is the prevailing form associated with PSII in its nonphosphorylated state (41). Based on the light-stimulated phosphorylation of isolated LHCII before resolution into monomers and trimers and on the specific radioactivity of the 32P-γ-ATP, one can calculate incorporation of 5 nmols 32P μmol chl−1 of LHCII. Assuming 14 chl molecules per LHCII monomer (42), one can estimate an average incorporation of 70 nmols 32P μmol chl−1 of LHCII monomers. The phosphorylation of the LHCII trimers is about 5-fold higher than that of the monomer (Fig. 3) and thus, about one phosphate is incorporated/LHCII trimer in the in vitro system, which is equivalent to 30% of the maximum expected if one phosphothreonine site can be phosphorylated per monomer (33, 43). This phosphorylation level is similar to that obtained in isolated thylakoids during state transition (2), indicating the similarity of the in vitro reconstituted and in situ systems.
The light-induced conformational changes of the nonphosphorylated LHCII may alter the interaction between PSII and LHCII trimers, concomitantly exposing the LHCII N-terminal domain to the kinase. Phosphorylation then may stabilize the new conformation of the LHCII and destabilize the PSII-LHCII trimer interaction, leading to their dissociation.
Phosphorylation of LHCII in situ can be induced by light-independent reduction of the plastoquinone pool and the cytochrome bf complex (2, 15). Thus, the light-induced exposure of the LHCII phosphorylation sites in situ may affect the initial kinetics and efficiency of the process. However, considering that dephosphorylation of phospho-LHCII by the thylakoid phosphatase occurs concomitant with the phosphorylation process (44, 45), the steady-state level of the phosphorylated LHCII in vivo depends on its phosphorylation and dephosphorylation rates and one should consider that the latter process may be affected by the light-induced conformational change of LHCII as well.
Conclusions.
The results obtained in this work, using an in vitro kinase-LHCII substrate reconstituted system as well as isolated thylakoids, indicate that illumination affects the conformation of the chl-protein complex and points toward a novel aspect of the light effect on the dynamics of photosynthetic membrane protein complexes.
Acknowledgments
We dedicate this article to Dr. Alma Gal, who contributed substantially to the initial development of this research project in the Jerusalem laboratory and lost a long-lasting battle against a merciless disease in January 1998. We are grateful to G. Hauska (Regensburg) for his gift of cytochrome f and Rieske protein antibodies and to T. Kuwabara (Tsukuba) for polyphenol-oxidase antibodies. We acknowledge the support by The Human Frontiers Science Program, The Sonderforschungsbereiche 184, The Deutsche Forschungsgemeinschaft, The Israeli Science Foundation-Israeli Academy of Sciences, The Swedish Research Council for Agriculture and Forestry, The Nobel Committee for Chemistry, The German-Israel Science Foundation (GIF) awarded to I.O. and H.P., and the European Molecular Biology Organization for a Short-Term Fellowship to H.G.D.-H. for time spent in I.O.’s laboratory.
ABBREVIATIONS
- chl
chlorophyll
- DCMU
3-(3,4-dichlorophenyl)-1,1-dimethyl-urea
- LHCII
light-harvesting complex II
- PS
photosystem
References
- 1.Allen J F. Biochim Biophys Acta. 1992;1098:275–335. doi: 10.1016/s0005-2728(09)91014-3. [DOI] [PubMed] [Google Scholar]
- 2.Gal A, Zer H, Ohad I. Physiol Plant. 1997;100:869–885. [Google Scholar]
- 3.Carlberg I, Andersson B. Photosynth Res. 1996;47:145–156. doi: 10.1007/BF00016177. [DOI] [PubMed] [Google Scholar]
- 4.Elich T D, Edelman M, Mattoo A K. FEBS Lett. 1997;411:236–238. doi: 10.1016/s0014-5793(97)00698-4. [DOI] [PubMed] [Google Scholar]
- 5.Hammer M F, Markwell J, Sarath G. Plant Physiol. 1997;113:227–233. doi: 10.1104/pp.113.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fulgosi H, Vener A V, Altschmied L, Herrmann R G, Andersson B. EMBO J. 1998;17:1577–1587. doi: 10.1093/emboj/17.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vener A V, Ohad I, Andersson B. Curr Opin Plant Biol. 1998;1:217–223. doi: 10.1016/s1369-5266(98)80107-6. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson A, Stys D, Drakenberg T, Spangfort M D, Forsén S, Allen J F. J Biol Chem. 1997;272:18350–18357. doi: 10.1074/jbc.272.29.18350. [DOI] [PubMed] [Google Scholar]
- 9.Keren N, Ohad I. In: Advances in Photosynthesis—Molecular Biology of Chlamydomonas: Chloroplast and Mitochondria. Rochaix J D, Goldscmidt-Clermont M, Merchant S, editors. Vol. 7. Dordrecht, The Netherlands: Kluwer; 1998. pp. 569–596. [Google Scholar]
- 10.Staehelin L A, Arntzen C J. J Cell Biol. 1983;97:1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallon O, Bulté L, Dainese P, Olive J, Bassi R, Wollman F-A. Proc Natl Acad Sci USA. 1991;88:8262–8266. doi: 10.1073/pnas.88.18.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett J, Shaw E K, Michel H P. Eur J Biochem. 1988;171:95–100. doi: 10.1111/j.1432-1033.1988.tb13763.x. [DOI] [PubMed] [Google Scholar]
- 13.Gal A, Schuster G, Frid D, Canaani O, Schwieger H G, Ohad I. J Biol Chem. 1988;263:7785–7791. [PubMed] [Google Scholar]
- 14.Lemaire C, Girard-Bascou J, Wollman F-A. In: Progress in Photosynthetic Research. Biggins J, editor. IV. Dordrecht, The Netherlands: Martinus Nijhoff; 1986. pp. 655–658. [Google Scholar]
- 15.Vener A V, van Kan P J M, Rich P R, Ohad I, Andersson B. Proc Natl Acad Sci USA. 1997;94:1585–1590. doi: 10.1073/pnas.94.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlberg I, Rintamäki E, Aro E-M, Andersson B. Biochemistry. 1999;38:3197–3204. doi: 10.1021/bi982506o. [DOI] [PubMed] [Google Scholar]
- 17.Hauska G. Methods Enzymol. 1986;126:271–285. [Google Scholar]
- 18.Gal A, Hauska G, Herrmann R G, Ohad I. J Biol Chem. 1990;265:19742–19749. [PubMed] [Google Scholar]
- 19.Gal A, Zer H, Roobol-Boza M, Fulgosi H, Herrmann R G, Ohad I, Andersson B. In: Photosynthesis: From Light to Biosphere. Mathis P, editor. III. Dordrecht, The Netherlands: Kluwer; 1995. pp. 341–344. [Google Scholar]
- 20.Regnier F E. Nature (London) 1991;350:634–635. doi: 10.1038/350634a0. [DOI] [PubMed] [Google Scholar]
- 21.Roobol-Boza M, Shochat S, Tjus S E, Hagman Å, Gast P, Andersson B. Photosynth Res. 1995;46:339–345. doi: 10.1007/BF00020449. [DOI] [PubMed] [Google Scholar]
- 22.Sokolenko A, Fulgosi H, Gal A, Altschmied L, Ohad I, Herrmann R G. FEBS Lett. 1995;371:176–180. doi: 10.1016/0014-5793(95)00892-d. [DOI] [PubMed] [Google Scholar]
- 23.Schuster G, Timberg R, Ohad I. Eur J Biochem. 1988;177:403–410. doi: 10.1111/j.1432-1033.1988.tb14389.x. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Paulsen H, Hobe S. Eur J Biochem. 1992;205:71–76. doi: 10.1111/j.1432-1033.1992.tb16752.x. [DOI] [PubMed] [Google Scholar]
- 26.Rintamäki E, Salonen M R, Suoranta U-M, Carlberg I, Andersson B, Aro E-M. J Biol Chem. 1997;272:30476–30482. doi: 10.1074/jbc.272.48.30476. [DOI] [PubMed] [Google Scholar]
- 27.Mullet J E, Arntzen C J. Biochim Biophys Acta. 1980;589:100–117. doi: 10.1016/0005-2728(80)90135-8. [DOI] [PubMed] [Google Scholar]
- 28.Hobe S, Förster R, Klinger J, Paulsen H. Biochemistry. 1995;34:10224–10228. doi: 10.1021/bi00032a016. [DOI] [PubMed] [Google Scholar]
- 29.Kirilovsky D, Kessel M, Ohad I. Biochim Biophys Acta. 1983;1724:416–426. [Google Scholar]
- 30.Barber J, Nield J, Morris E, Zheleva D, Hankamer B. Physiol Plant. 1997;100:817–827. [Google Scholar]
- 31.Bennett J. Nature (London) 1979;269:344–346. [Google Scholar]
- 32.Schuster G, Dewit M, Staehelin L A, Ohad I. J Cell Biol. 1986;103:71–80. doi: 10.1083/jcb.103.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett J. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:281–311. [Google Scholar]
- 34.Burgi R, Suter F, Zuber H. Biochim Biophys Acta. 1987;890:346–351. [Google Scholar]
- 35.Horton P, Ruban A V, Walters R G. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 36.Barzda V, Istokovics A, Simidjiev I, Garab G. Biochemistry. 1996;35:8981–8985. doi: 10.1021/bi960114g. [DOI] [PubMed] [Google Scholar]
- 37.Garab G, Leegood R C, Walker D A, Sutherland J C, Hind G. Biochemistry. 1988;27:2430–2434. [Google Scholar]
- 38.Garab G, Istokovics A, Butiuc A, Simidjiev I, Der A. In: Photosynthesis: Mechanisms and Effects. Garab G, editor. Dordrecht, The Netherlands: Kluwer; 1998. pp. 341–347. [Google Scholar]
- 39.Barber J. In: Photosynthesis III: Photosynthetic Membranes and Light Harvesting Systems. Staehelin L A, Arntzen C J, editors. Vol. 19. Berlin: Springer; 1986. pp. 653–663. [Google Scholar]
- 40.Allen J F, Nilsson A. Physiol Plant. 1997;100:863–868. [Google Scholar]
- 41.Jansson S. Biochim Biophys Acta. 1994;1184:1–19. doi: 10.1016/0005-2728(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 42.Kühlbrandt W, Wang D N, Fujiyoshi Y. Nature (London) 1994;367:614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- 43.Owens G C, Ohad I. J Biol Chem. 1982;93:712–718. doi: 10.1083/jcb.93.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett J. Eur J Biochem. 1980;104:85–89. doi: 10.1111/j.1432-1033.1980.tb04403.x. [DOI] [PubMed] [Google Scholar]
- 45.Silverstein T, Cheng L, Allen J F. FEBS Lett. 1993;334:101–105. doi: 10.1016/0014-5793(93)81690-2. [DOI] [PubMed] [Google Scholar]