Abstract
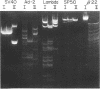
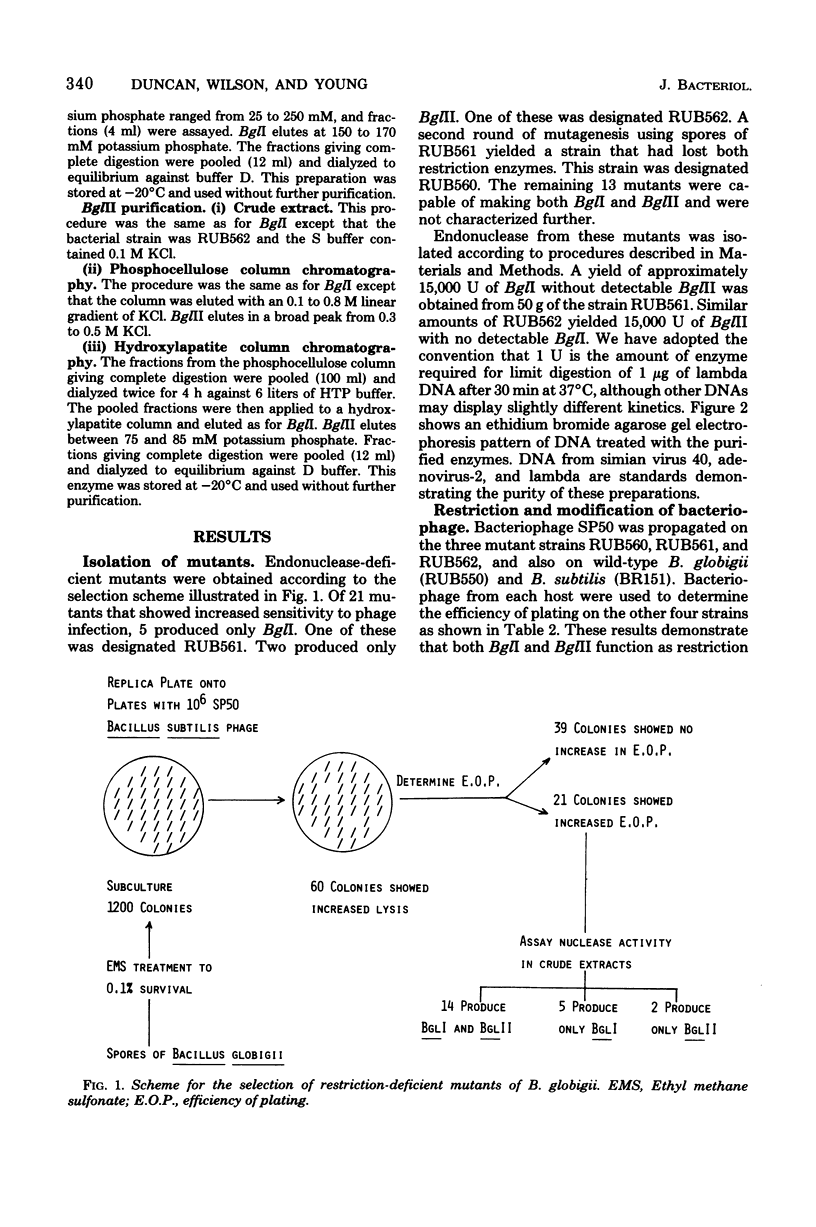
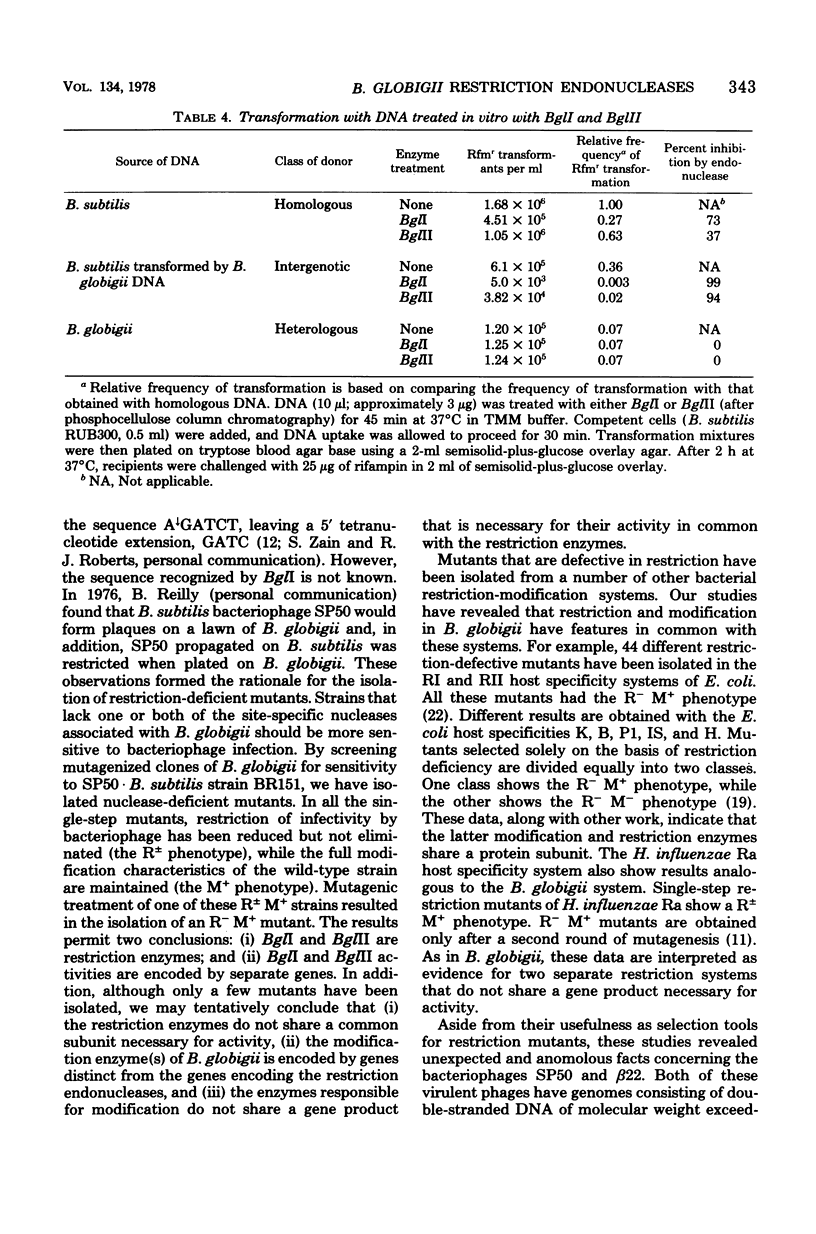
Bacillus globigii contains two site-specific endonucleases, BPGLI AND BglI. A rapid technique for selection of mutants deficient in each of these enzymes was developed using sensitivity to infection by bacteriophage SP50 as an indication of the levels of enzyme. Mutants defective in BglI, BglII, and both BglI and BglII retained the wild-type modification phenotype. Genetic and biochemical studies have established that these enzymes are involved in restriction in vivo. Simplified purification procedures for BglI and BglII using these mutants are described.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biswal N., Kleinschmidt A. K., Spatz H. C., Trautner T. A. Physical properties of the DNA of bacteriophage SP50. Mol Gen Genet. 1967;100(1):39–55. doi: 10.1007/BF00425774. [DOI] [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H., Brooks D., Young F. E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972 Apr;110(1):281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall J. F., Welker N. E. Isolation and properties of a thermostable restriction endonuclease (ENDO R-Bst1503). J Bacteriol. 1977 Feb;129(2):1110–1120. doi: 10.1128/jb.129.2.1110-1120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton M. D., McCarthy B. J. Genetic and base sequence homologies in bacillus. Genetics. 1969 Jul;62(3):697–710. [PMC free article] [PubMed] [Google Scholar]
- Duncan C. H., Wilson G. A., Young F. E. Transformation of Bacillus subtilis and Escherichia coli by a hybrid plasmid pCD1. Gene. 1977 Mar;1(2):153–167. doi: 10.1016/0378-1119(77)90026-9. [DOI] [PubMed] [Google Scholar]
- FOELDES J., TRAUTNER T. A. INFECTIOUS DNA FROM A NEWLY ISOLATED B. SUBTILIS PHAGE. Z Vererbungsl. 1964 Apr 10;95:57–65. doi: 10.1007/BF00898184. [DOI] [PubMed] [Google Scholar]
- Havender W. R., Trautner T. A. Genetic and transfection studies with B. subtilis phage SP50. 3. Biological effects of DNA cleavage and the physical basis of the map. Mol Gen Genet. 1972;116(1):51–67. doi: 10.1007/BF00334260. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Nucleic acid synthesis in Bacillus subtilis infected with bacteriophage beta-22. J Virol. 1970 Oct;6(4):381–392. doi: 10.1128/jvi.6.4.381-392.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Spizizen J. Increased rate of asporogenous mutations following treatment of Bacillus subtilis spores with ethyl methanesulfonate. Mutat Res. 1971 Sep;13(1):93–96. doi: 10.1016/0027-5107(71)90130-8. [DOI] [PubMed] [Google Scholar]
- Piekarowicz A., Glover S. W. Host specificity of DNA in Haemophilus influenzae: the two restriction and modification systems in strain Ra. Mol Gen Genet. 1972;116(1):11–25. doi: 10.1007/BF00334255. [DOI] [PubMed] [Google Scholar]
- Pirrotta V. Two restriction endonucleases from Bacillus globiggi. Nucleic Acids Res. 1976 Jul;3(7):1747–1760. doi: 10.1093/nar/3.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- Spatz H. C., Trautner T. A. The role of recombination in transfection of B. subtilis. Mol Gen Genet. 1971;113(2):174–190. doi: 10.1007/BF00333191. [DOI] [PubMed] [Google Scholar]
- Trautner T. A., Pawlek B., Bron S., Anagnostopoulos C. Restriction and modification in B. subtilis. Biological aspects. Mol Gen Genet. 1974;131(3):181–191. doi: 10.1007/BF00267958. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Intergenotic transformation of the Bacillus subtilis genospecies. J Bacteriol. 1972 Sep;111(3):705–716. doi: 10.1128/jb.111.3.705-716.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis: evidence for selective induction of prophage in competent cells. J Bacteriol. 1975 Jan;121(1):296–304. doi: 10.1128/jb.121.1.296-304.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehle C. O., Doi R. H. Differential expression of bacteriophage genomes in vegetative and sporulating cells of Bacillus subtilis. J Virol. 1967 Oct;1(5):935–947. doi: 10.1128/jvi.1.5.935-947.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori R., Roulland-Dussoix D., Boyer H. W. R factor-controlled restriction and modification of deoxyribonucleic acid: restriction mutants. J Bacteriol. 1972 Dec;112(3):1275–1279. doi: 10.1128/jb.112.3.1275-1279.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]