Abstract
Medical advances in the management of patients with sickle cell disease, thalassemia, and other hemolytic anemias have led to significant increases in life expectancy. Improved public health, neonatal screening, parental and patient education, advances in red cell transfusion medicine, iron chelation therapy, penicillin prophylaxis for children, pneumococcal immunization, and hydroxyurea therapy have all likely contributed to this effect on longevity.1,2 Importantly, as a generation of patients with sickle cell disease and thalassemia ages, new chronic complications of these hemoglobinopathies develop. In this context, pulmonary hypertension is emerging as one of the leading causes of morbidity and mortality in adult sickle cell and thalassemia patients, and likely in patients with other hemolytic anemias. A common feature of both sickle cell disease and thalassemia is intravascular hemolysis and chronic anemia. Recent data suggest that chronic intravascular hemolysis is associated with a state of endothelial dysfunction characterized by reduced nitric oxide (NO) bioavailability, pro-oxidant and pro-inflammatory stress and coagulopathy, leading to vasomotor instability and ultimately producing a proliferative vasculopathy, a hallmark of which is the development of pulmonary hypertension in adulthood.3–5 In conclusion, pulmonary hypertension is common in patients with hereditary hemolytic anemias and is associated with a high risk of death in patients with sickle cell disease. New therapies targeting this vasculopathy and aimed at normalizing the vasodilator:vasoconstrictor balance are discussed.
Endothelial Control of Vascular Function
No is a soluble diatomic gas molecule, much like carbon monoxide. Because of its unpaired electron, NO is a free radical, providing it with unique reactivities and biological properties.6 NO is produced in endothelium by the endothelial NO synthase enzyme, by an oxygen-dependent conversion of l-arginine to citrulline.7 Once produced, NO can diffuse in a paracrine fashion to adjacent smooth muscle, where it binds avidly to the heme moiety of soluble guanylate cyclase. This activates the enzyme, which in turn converts GTP to cGMP, activating cGMP-dependent protein kinases, which ultimately sequester calcium and produce vasodilation.8,9 In addition to this vasodilation, which is tonic in nature and controls approximately 25% of our resting blood flow, NO promotes general vascular homeostasis (Table 1). Importantly, NO also reacts with the oxygenated and deoxygenated heme groups of hemoglobin at nearly diffusion limited rates (107 M-1 sec-1) to produce methemoglobin and nitrate, and iron-nitrosyl-hemoglobin, respectively (Equation 1 and Equation 2).10
Table 1.
Vascular effects of nitric oxide
| Vasodilatory |
| Relaxation of vascular smooth muscle |
| Decreased expression of endothelin-1 and endothelin |
| receptor |
| Anti-adhesive |
| Decreased endothelial expression of adhesion molecules |
| Anti-thrombotic |
| Decreased platelet activation |
| Decreased tissue factor activity |
| Decreased thrombin generation |
| Anti-oxidant |
| Inactivation of reactive oxygen species |
| Equation 1 |
| Equation 2 |
Because these reactions are so fast and, in the case of Equation 1, irreversible, kinetic calculations would predict that NO produced from endothelium would not survive long enough to diffuse to smooth muscle, becoming inactivated by rapid reaction with intravascular hemoglobin (10 mM heme concentration in whole blood).11 This paradox of biology is solved by the physical properties of the erythrocyte, of laminar flowing blood, and the plasma hemoglobin scavenging systems that limit extravasation of free plasma hemoglobin into the interstitial space. During normal physiology, the reaction of NO with hemoglobin is limited by compartmentalization of hemoglobin within the erythrocyte membrane.12 This compartmentalization of hemoglobin from endothelium creates two diffusional barriers: a cell-free diffusion barrier along the endothelium in laminar flowing blood13,14 and an unstirred bulk diffusional barrier around the erythrocyte membrane.15 Understanding the role of such barriers and the requirement for a physical separation of hemoglobin from the source of NO production in endothelium helps explain the remarkable morbidity and mortality associated with the use of stroma-free hemoglobin-based blood substitutes and many of the clinical manifestations of hemolytic disease.3,4,10
Endothelial Dysfunction in Sickle Cell Disease: A Unique State of NO Resistance
In patients who have coronary artery disease, atherosclerosis and its risk factors (obesity, hypertension, diabetes, tobacco smoking, and hypercholesterolemia) a state of endothelial dysfunction is observed, characterized by decreased function of endothelial NO synthase (Figure 1; see color figures, page 546). In patients with sickle cell disease there is a similar dysfunction, characterized by a blunted response to NO synthase inhibition. Unlike patients with atherosclerosis, however, there is also a resistance to exogenously delivered NO donors. This has been demonstrated by multiple investigators in both human and transgenic mouse studies:
Our group has shown that the blood flow responses to infusions of the NO synthase inhibitor l-NMMA are blunted and that blood flow responses to the NO donor sodium nitroprusside are nearly abolished in patients with high plasma hemoglobin concentrations.3,16
Eberhardt and colleagues have shown that endothelium-dependent, NO-dependent blood flow is impaired in patients with sickle cell disease, when measured by flow-mediated vasodilation. They also showed that responses to the exogenous NO donor, nitroglycerin, are impaired, compared to control subjects with nonhemolytic anemia.17
Both Nath et al and Kaul et al have described a similar state of resistance to exogenous NO (the NO donor NONOate or sodium nitroprusside) in different transgenic mouse models of sickle cell disease.18,19 Kaul and colleagues recently demonstrated that this state of NO resistance correlated with plasma hemoglobin levels and suggested that NO resistance in this model was linked to hemolytic rate and oxidant stress.5
Aslan and Freeman have shown that NO is inhibited in the vasculature of transgenic sickle cell mice with sickle cell disease by a diffusion-limited reaction with superoxide produced from xanthine oxidase on endothelium.20,21 Increased xanthine oxidase expression in the lung of the transgenic mouse has also been reported to scavenge NO in this vascular system.22 Recent studies have suggested a role for vascular NADPH oxidase in aberrant superoxide-mediated NO scavenging in the sickle cell cerebral vasculature.23
It is increasingly clear from these studies that plasma hemoglobin-mediated and oxygen free radical–mediated consumption of NO produces a state of resistance to NO in patients with sickle cell disease. During intravascular hemolysis, the diffusional barriers that limit NO reactions with hemoglobin are disrupted and the cell-free plasma hemoglobin destroys NO at a rate 1000-fold faster than intra-erythrocytic hemoglobin.4,12,24 Consequently, smooth muscle guanylyl cyclase is not activated and vasodilation is impaired. In support of this mechanism, plasma from patients with sickle cell disease contains cell-free ferrous oxy-hemoglobin, which stoichiometrically consumes micromolar quantities of NO and abrogates forearm blood flow responses to NO donor infusions.3
Downstream effects of intravascular hemolysis and NO consumption include increased endothelin-1 expression, heme and free iron mediated oxygen radical generation, platelet activation and increased endothelial adhesion molecule expression (recently reviewed in4). In patients with sickle cell disease, plasma endothelin-1 levels are increased in steady state and during crisis.25–27 In vitro, sickle erythrocytes increase endothelin-1 production by cultured human endothelial cells and endothelin receptor A antagonism decreases the vasoconstrictive effects of conditioned media from pulmonary endothelial cells exposed to sickled erythrocytes on aortic rings.28 In addition, endothelin-1 activates Gardos channels in human sickle erythrocytes, an effect that may promote sickle cell dehydration and facilitate red blood cell sickling and adhesion.29
Intravascular hemolysis has the potential to drive a pro-coagulant state. Platelet activation is profoundly inhibited by NO and such NO-dependent inhibition may in turn be blocked by plasma hemoglobin-mediated NO scavenging.30 Additionally, hemolytic rate (reticulocytosis) is associated with hemoglobin desaturation (ventilation/perfusion inhomogeneiety) and adhesion molecule expression;31 it is possible that such a hypoxic state can induce hypoxia-inducing factor-1 (HIF-1) dependent factors such as erythropoietin, vascular endothelial growth factor (VEGF), and endothelin-1.
In addition to release of hemoglobin from the red cell into plasma, hemolysis releases erythrocyte arginase, which converts l-arginine, the substrate for NO synthesis, to ornithine.32 Morris and colleagues found that arginase activities in the plasma of patients correlated significantly with plasma hemoglobin and LDH and was increased in the plasma and red cells of patients with sickle cell disease. Consistent with this observation, in patients with sickle cell disease, the arginine-to-ornithine ratio decreases significantly as pulmonary pressures increase and was independently associated with increasing mortality.32,33 Arginine therapy has been shown to decrease pulmonary pressures in patients with sickle cell disease and secondary pulmonary hypertension34 and has been shown to inhibit endothelin-1 mediated activation of the Gardos channel in the transgenic sickle cell mouse and thus limit erythrocyte dehydration.35
These mechanisms likely contribute to the progressive development of sickle cell vasculopathy, characterized by vasoconstriction, intimal and smooth muscle hyperplasia and in situ thrombosis (Figure 2; see color figures, page 546).
Does hemolysis produce a subset of clinical manifestations shared by the hereditary and acquired hemolytic anemias?
We have proposed that the clinical manifestations of sickle cell disease may fall into two partially overlapping subphenotypes. The first subphenotype encompasses the more classic clinical manifestations of the disease: vasoocclusive pain crisis and the acute chest syndrome. These clinical morbidities are epidemiologically associated with high white blood cell counts, high steady state hemoglobin levels and low fetal hemoglobin levels (increasing fetal hemoglobin concentration is protective).36 These “vasoocclusive” complications are widely presumed to be mediated by microvascular obstruction by sickle erythrocytes and the pathogenesis characterized by ischemia-reperfusion injury, infarction and inflammation.37,38 The second subphenotype encompasses clinical complications shared by other hemolytic anemias (Table 2) and includes pulmonary arterial hypertension, systemic systolic arterial hypertension, cutaneous leg ulceration, priapism and possibly stroke.3,33,39 Pulmonary hypertension is increasingly observed in hemolytic anemias (Table 2), including sickle cell disease33,40,41 and thalassemia (in particular thalassemia intermedia, such as Hb E-β0-thalassemia, and inadequately transfused and chelated patients with thalassemia major).42,43 In addition to published case reports of pulmonary hypertension in diseases listed in Table 2 (this extensive list of diseases associated with pulmonary hypertension is not cited due to space limitations, but a pubmed search using the search terms “anemia” and “pulmonary hypertension” will identify reports), our group has received additional reports of patients with pulmonary hypertension associated with hemolytic anemia secondary to unstable hemoglobin variants (personal communication, H. Franklin Bunn and Thomas DeLoughery).
Table 2.
Conditions associated with both intravascular hemolysis and increased risk for pulmonary hypertension
| Hereditary hemolytic anemia |
| Sickle cell disease |
| Thalassemia |
| Hereditary spherocytosis |
| Hereditary stomatocytosis |
| Pyruvate kinase deficiency |
| Unstable hemoglobin variants |
| Acquired hemolytic anemia |
| Microangiopathic hemolytic anemias |
| Paroxysmal nocturnal hemoglobinuria |
| Schistosomiasis |
| Mechanical heart valves |
| Left ventricular assist devices |
| Cardiopulmonary bypass devices |
| Malaria (?) |
Pulmonary Arterial Hypertension in Sickle Cell Disease
Prevalence
Echocardiographic studies have reported that approximately 30% of screened adult patients with sickle cell anemia have pulmonary hypertension (systolic pulmonary artery pressures (PAP) ≥ 30 mm Hg).33,41,44,45 Recent autopsy studies suggest that up to 75% of sickle cell patients have histological evidence of pulmonary arterial hypertension at the time of death.46 Similarly, retrospective studies have demonstrated that 40%–50% of patients with thalassemia intermedia,43 and 10%–75% of patients with thalassemia major, have echocardiographic evidence of pulmonary hypertension.47,48
Risk factors
In the NIH pulmonary hypertension screening study, all markers of hemolytic anemia, including low hemoglobin and hematocrit, high lactate dehydrogenase (LDH), and high aspartate aminotransferase, but not alanine aminotransferase levels, were associated with elevated pulmonary pressures.33 Multiple logistic regression analysis identified a history of renal or cardiovascular complications, increased systemic systolic blood pressure, LDH, elevated alkaline phosphatase, and low transferrin levels as independent predictors of pulmonary hypertension. In men, a history of priapism was an additional independent factor associated with pulmonary hypertension. These associated risk factors for pulmonary hypertension suggest that pulmonary hypertension represents one element of the systemic vasculopathy seen in some patients with sickle cell disease (systemic hypertension, renal failure and priapism) and is mechanistically linked to hemolytic rate, iron overload and cholestatic hepatic dysfunction. Interestingly, the development of pulmonary hypertension was not associated with markers of inflammation, fetal hemoglobin levels or platelet counts.
Functional or surgical asplenia may also contribute to the development of pulmonary hypertension in patients with hemolytic disorders. Splenectomy has been reported to be a risk factor for the development of pulmonary hypertension, particularly in patients with hemolytic disorders.49–53 It has been speculated that the loss of splenic function increases the circulation of platelet derived mediators and that senescent and abnormal erythrocytes in the circulation trigger platelet activation, promoting pulmonary microthrombosis and red cell adhesion to the endothelium.49 Intravenous injection of hemolysate promotes the formation of platelet-rich thrombi in the pulmonary vascular bed of rabbits after ligation of the splenic artery, without any thrombus formation in the animals without splenic artery ligation.54 A role for intensification of intravascular hemolysis following splenectomy (in contrast to predominantly spleen-mediated extravascular hemolysis) has also been suggested by the demonstration of significantly higher plasma hemoglobin and erythrocyte-derived microvesicle levels in patients with thalassemia intermedia who have undergone splenectomy, compared with those who have not.55
Interestingly, the number of episodes of acute chest syndrome (a potential cause of chronic lung disease and pulmonary fibrosis) was not associated with pulmonary hypertension in our prospective prevalence study.33 In addition, a similar prevalence of pulmonary hypertension in patients with thalassemia intermedia, who do not develop the acute chest syndrome, suggests that acute lung injury may worsen pulmonary hypertension but certainly is not etiologic. In our cohort, individuals with pulmonary hypertension have a higher incidence of restrictive lung disease and pulmonary fibrosis on high-resolution chest computed tomography (CT) than age- and hemoglobin-matched patients with sickle cell disease without pulmonary hypertension (Anthi et al, 2005, manuscript under review). Further, in patients with thalassemia, restrictive ventilatory defects and pulmonary fibrosis—associated with pulmonary hypertension—have also been documented.56,57 Taken together, these data suggest that similar pathogenic proliferative mechanisms that lead to pulmonary hypertension may underlie the genesis of pulmonary fibrosis in these patients.
Diagnosis
Doppler echocardiography provides essential information such as non-invasive estimation of pulmonary artery systolic pressure (via calculation of the tricuspid regurgitant Doppler jet velocity value [TRV]), valvular function and right and left ventricular function. The use of echocardiography to estimate pulmonary artery systolic pressures has been validated in patients with sickle cell disease, and noninvasive assessment correlates well with the measurement of pulmonary arterial pressures by right heart catheterization.33 The velocity of regurgitant blood across the tricuspid valve during systole is measured, and the pulmonary artery systolic pressure is calculated using the modified Bernoulli’s equation [(4 × TRV²) plus central venous pressure estimate]; method described in detail in 33). To avoid the more subjective estimation of central venous pressures, pulmonary hypertension can be defined by a specific TRV ≥ 2.5 m/sec (based on high risk of death using this value in a prospective cohort study33) and moderate-to-severe pulmonary hypertension defined by a TRV ≥ 3.0 m/sec (the more conventional criteria which is consistent with a pulmonary artery systolic pressure of at least 41 mm Hg). The significance of these values has only been defined in adult patients with sickle cell disease; limited information is available for children.
Prognosis
Patients with sickle cell disease and pulmonary hypertension have a significantly increased mortality rate compared with patients without pulmonary hypertension. Sutton and colleagues reported a 40% mortality rate at 22 months with an odds ratio for death of 7.86 (2.63–23.4).44 Powars and colleagues reported a mean 2.5-year survival in sickle cell patients with chronic lung disease with pulmonary hypertension.58 Castro and colleagues40 similarly reported a 50% 2-year mortality rate in patients with sickle cell disease with pulmonary hypertension confirmed by right heart catheterization.
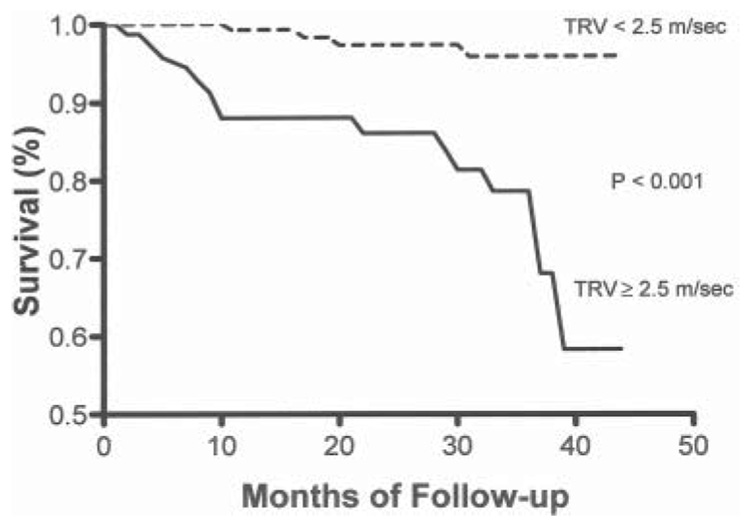
Consistent with retrospective studies indicating that pulmonary hypertension is associated with a higher mortality, in the NIH screening study a measured TRV of at least 2.5 m/sec, as compared to a velocity of less than 2.5 m/sec, was associated with a marked increased risk of death (RR 10.1; 95% CI, 2.2–47; P < 0.001) and remained so after adjustment for other possible risk factors in proportional hazards regression analysis. The 18-month mortality was 16% for patients with a TRV of greater than or equal to 2.5 m/sec and was less than 2% in patients without pulmonary hypertension. Further updated follow-up data from this cohort continue to demonstrate that pulmonary hypertension is a strong independent risk factor for mortality (RR 7.4, 95% CI 2.4–22.6, P < 0.001) with 40-month mortality rate of approximately 40% (Figure 3). In addition, De Castro and colleagues reported a remarkably similar 17% mortality rate for patients with pulmonary hypertension over 2 years compared with approximately 2% for subjects without pulmonary hypertension.59 Taken together, the retrospective40,44,58 and prospective33,41 studies strongly support the contention that pulmonary hypertension is the greatest risk factor facing the aging population of patients with sickle cell disease and likely other patients with chronic high-grade intravascular hemolysis.
Figure 3. Kaplan-Meier survival curves according to the tricuspid regurgitant jet velocity (TRV).
The survival rate is significantly higher among patients with a TRV of less than 2.5 m per second than among those with a TRV of at least 2.5 m per second (P < 0.001). Updated from Gladwin et al. April 2005.33
Management
In the absence of clinical guidelines and placebo-controlled therapeutic trials for the evaluation and treatment of pulmonary hypertension in the sickle cell population, we now summarize our empiric and anecdotal diagnostic and therapeutic approach for the adult patient with sickle cell disease diagnosed with pulmonary hypertension. Because we do not yet know if an elevated pulmonary pressure is a direct cause of death or a risk factor for multi-organ disease and generalized sickle cell vasculopathy, for patients with mild pulmonary hypertension (TRV 2.5–2.9 m/s) we recommend intensification of sickle cell-specific therapy.
Consider hydroxyurea treatment at the maximum tolerated dose as defined by the Multicenter Study of Hydroxyurea, with erythropoietin therapy considered if reticulocytopenia limits hydroxyurea therapy.
Monthly transfusion therapy may be considered for patients with poor responses to hydroxyurea, accompanied by chelation therapy, if indicated. Anecdotally, the TRV has declined in some patients with institution of these treatment measures, although this has not been studied to date.
Consultation may be considered with a pulmonologist or cardiologist experienced in pulmonary hypertension, the latter especially if the echocardiogram shows evidence of left ventricular dysfunction.
Identify and treat risk factors associated with pulmonary hypertension such as hypoxemia during rest or exercise and nocturnal hypoxemia, sleep apnea, pulmonary thromboembolic disease, left ventricular systolic and diastolic dysfunction, severe anemia and iron-overload.
In addition to the above measures, we recommend that patients with TRV ≥ 3 m/s should undergo:
Right heart catheterization to assess left ventricular diastolic and systolic function.
A CT-pulmonary angiogram to exclude chronic thromboembolic pulmonary hypertension.
Consider systemic anticoagulation. Therapy with warfarin improves outcomes in patients with primary pulmonary hypertension and in-situ thrombosis but no data are available in patients with sickle cell disease.
Consider specific therapy with selective pulmonary vasodilator and remodeling drugs, particularly if the patient has symptomatic dyspnea on exertion that has progressed in recent months or years. Drugs that are FDA-approved for primary pulmonary hypertension include bosentan (Tracleer®) and various forms of prostaglandin therapy, none of which have been comprehensively investigated for sickle cell pulmonary hypertension. We have pilot experience with sildenafil, which has recently gained FDA approval for pulmonary hypertension under the trade name Revatio®.60 Two multicenter trials using sildenafil and bosentan, for hemolysis-associated pulmonary hypertension are anticipated in the near future. Appropriate consultation and right heart catheterization are recommended at baseline and should be repeated annually. More detailed management recommendations are available in recently published reviews.61–63
Conclusions
In patients with sickle cell disease, and likely other hemolytic conditions, intravascular hemolysis produces a state of endothelial dysfunction characterized by reduced NO bioavailability and NO resistance. This leads to dysregulation of the endothelium-derived vasodilator:vasoconstrictor system leading to acute vasoconstriction and chronic proliferative vasculopathy. We propose that this vasculopathy is characterized epidemiologically by a clinical subphenotype of pulmonary hypertension, cutaneous leg ulceration, priapism, sudden death, and possibly stroke. Pulmonary hypertension is common in patients with hereditary hemolytic anemias and is associated with a high risk of death in patients with sickle cell disease. New therapies targeting this vasculopathy and aimed at normalizing the vasodilator:vasoconstrictor balance are in therapeutic trial.
References
- 1.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337:762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 3.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 4.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. Jama. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 5.Kaul DK, Liu XD, Chang HY, Nagel RL, Fabry ME. Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice. J Clin Invest. 2004;114:1136–1145. doi: 10.1172/JCI21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wink DA, Mitchell JB. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med. 1998;25:434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 7.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 8.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′- cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 10.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. No scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36:685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Lancaster JR., Jr A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide. 1997;1:18–30. doi: 10.1006/niox.1996.0112. [DOI] [PubMed] [Google Scholar]
- 12.Schechter AN, Gladwin MT. Hemoglobin and the paracrine and endocrine functions of nitric oxide. N Engl J Med. 2003;348:1483–1485. doi: 10.1056/NEJMcibr023045. [DOI] [PubMed] [Google Scholar]
- 13.Liao JC, Hein TW, Vaughn MW, Huang KT, Kuo L. Intravascular flow decreases erythrocyte consumption of nitric oxide. Proc Natl Acad Sci U S A. 1999;96:8757–8761. doi: 10.1073/pnas.96.15.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaughn MW, Kuo L, Liao JC. Effective diffusion distance of nitric oxide in the microcirculation. Am J Physiol. 1998;274:H1705–H1714. doi: 10.1152/ajpheart.1998.274.5.H1705. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR., Jr Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 16.Gladwin MT, Schechter AN, Ognibene FP, et al. Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation. 2003;107:271–278. doi: 10.1161/01.cir.0000044943.12533.a8. [DOI] [PubMed] [Google Scholar]
- 17.Eberhardt RT, McMahon L, Duffy SJ, et al. Sickle cell anemia is associated with reduced nitric oxide bioactivity in peripheral conduit and resistance vessels. Am J Hematol. 2003;74:104–111. doi: 10.1002/ajh.10387. [DOI] [PubMed] [Google Scholar]
- 18.Nath KA, Shah V, Haggard JJ, et al. Mechanisms of vascular instability in a transgenic mouse model of sickle cell disease. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1949–R1955. doi: 10.1152/ajpregu.2000.279.6.R1949. [DOI] [PubMed] [Google Scholar]
- 19.Kaul DK, Liu XD, Fabry ME, Nagel RL. Impaired nitric oxide-mediated vasodilation in transgenic sickle mouse. Am J Physiol Heart Circ Physiol. 2000;278:H1799–H1806. doi: 10.1152/ajpheart.2000.278.6.H1799. [DOI] [PubMed] [Google Scholar]
- 20.Aslan M, Ryan TM, Adler B, et al. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci U S A. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aslan M, Ryan TM, Townes TM, et al. Nitric oxide-dependent generation of reactive species in sickle cell disease. Actin tyrosine induces defective cytoskeletal polymerization. J Biol Chem. 2003;278:4194–4204. doi: 10.1074/jbc.M208916200. [DOI] [PubMed] [Google Scholar]
- 22.Pritchard KA, Jr, Ou J, Ou Z, et al. Hypoxia-induced acute lung injury in murine models of sickle cell disease. Am J Physiol Lung Cell Mol Physiol. 2004;286:L705–L714. doi: 10.1152/ajplung.00288.2002. [DOI] [PubMed] [Google Scholar]
- 23.Wood KC, Hebbel RP, Granger DN. Endothelial cell NADPH oxidase mediates the cerebral microvascular dysfunction in sickle cell transgenic mice. FASEB J. 2005;19:989–991. doi: 10.1096/fj.04-3218fje. [DOI] [PubMed] [Google Scholar]
- 24.Gladwin MT, Lancaster JR, Jr, Freeman BA, Schechter AN. Nitric oxide’s reactions with hemoglobin: a view through the SNO-storm. Nat Med. 2003;9:496–500. doi: 10.1038/nm0503-496. [DOI] [PubMed] [Google Scholar]
- 25.Rybicki AC, Benjamin LJ. Increased levels of endothelin-1 in plasma of sickle cell anemia patients. Blood. 1998;92:2594–2596. [PubMed] [Google Scholar]
- 26.Graido-Gonzalez E, Doherty JC, Bergreen EW, Organ G, Telfer M, McMillen MA. Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood. 1998;92:2551–2555. [PubMed] [Google Scholar]
- 27.Hammerman SI, Kourembanas S, Conca TJ, Tucci M, Brauer M, Farber HW. Endothelin-1 production during the acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 1997;156:280–285. doi: 10.1164/ajrccm.156.1.9611085. [DOI] [PubMed] [Google Scholar]
- 28.Ergul S, Brunson CY, Hutchinson J, et al. Vasoactive factors in sickle cell disease: in vitro evidence for endothelin-1-mediated vasoconstriction. Am J Hematol. 2004;76:245–251. doi: 10.1002/ajh.20107. [DOI] [PubMed] [Google Scholar]
- 29.Rivera A, Rotter MA, Brugnara C. Endothelins activate Ca(2+)-gated K(+) channels via endothelin B receptors in CD-1 mouse erythrocytes. Am J Physiol. 1999;277:C746–C754. doi: 10.1152/ajpcell.1999.277.4.C746. [DOI] [PubMed] [Google Scholar]
- 30.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 31.Setty BN, Stuart MJ, Dampier C, Brodecki D, Allen JL. Hypoxaemia in sickle cell disease: biomarker modulation and relevance to pathophysiology. Lancet. 2003;362:1450–1455. doi: 10.1016/S0140-6736(03)14689-2. [DOI] [PubMed] [Google Scholar]
- 32.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 34.Morris CR, Morris SM, Jr, Hagar W, et al. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med. 2003;168:63–69. doi: 10.1164/rccm.200208-967OC. [DOI] [PubMed] [Google Scholar]
- 35.Romero JR, Suzuka SM, Nagel RL, Fabry ME. Arginine supplementation of sickle transgenic mice reduces red cell density and Gardos channel activity. Blood. 2002;99:1103–1108. doi: 10.1182/blood.v99.4.1103. [DOI] [PubMed] [Google Scholar]
- 36.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 37.Platt OS. Sickle cell anemia as an inflammatory disease [comment] J Clin Invest. 2000;106:337–338. doi: 10.1172/JCI10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J Clin Invest. 2000;106:411–420. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nolan VG, Wyszynski DF, Farrer LA, Steinberg MH. Hemolysis associated priapism in sickle cell disease. Blood. 2005 Jun 28; doi: 10.1182/blood-2005-04-1594. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–1261. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 41.Ataga KI, Sood N, De Gent G, et al. Pulmonary hypertension in sickle cell disease. Am J Med. 2004;117:665–669. doi: 10.1016/j.amjmed.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 42.Aessopos A, Farmakis D, Deftereos S, et al. Thalassemia heart disease: a comparative evaluation of thalassemia major and thalassemia intermedia. Chest. 2005;127:1523–1530. doi: 10.1378/chest.127.5.1523. [DOI] [PubMed] [Google Scholar]
- 43.Aessopos A, Farmakis D, Karagiorga M, et al. Cardiac involvement in thalassemia intermedia: a multicenter study. Blood. 2001;97:3411–3416. doi: 10.1182/blood.v97.11.3411. [DOI] [PubMed] [Google Scholar]
- 44.Sutton LL, Castro O, Cross DJ, Spencer JE, Lewis JF. Pulmonary hypertension in sickle cell disease. Am J Cardiol. 1994;74:626–628. doi: 10.1016/0002-9149(94)90760-9. [DOI] [PubMed] [Google Scholar]
- 45.Castro O. Systemic fat embolism and pulmonary hypertension in sickle cell disease. Hematol Oncol Clin North Am. 1996;10:1289–1303. doi: 10.1016/s0889-8588(05)70401-9. [DOI] [PubMed] [Google Scholar]
- 46.Haque AK, Gokhale S, Rampy BA, Adegboyega P, Duarte A, Saldana MJ. Pulmonary hypertension in sickle cell hemoglobinopathy: a clinicopathologic study of 20 cases. Hum Pathol. 2002;33:1037–1043. doi: 10.1053/hupa.2002.128059. [DOI] [PubMed] [Google Scholar]
- 47.Derchi G, Fonti A, Forni GL, et al. Pulmonary hypertension in patients with thalassemia major. Am Heart J. 1999;138:384. doi: 10.1016/s0002-8703(99)70129-8. [DOI] [PubMed] [Google Scholar]
- 48.Grisaru D, Rachmilewitz EA, Mosseri M, et al. Cardiopulmonary assessment in beta-thalassemia major. Chest. 1990;98:1138–1142. doi: 10.1378/chest.98.5.1138. [DOI] [PubMed] [Google Scholar]
- 49.Atichartakarn V, Likittanasombat K, Chuncharunee S, et al. Pulmonary arterial hypertension in previously splenectomized patients with beta-thalassemic disorders. Int J Hematol. 2003;78:139–145. doi: 10.1007/BF02983382. [DOI] [PubMed] [Google Scholar]
- 50.Chou R, DeLoughery TG. Recurrent thromboembolic disease following splenectomy for pyruvate kinase deficiency. Am J Hematol. 2001;67:197–199. doi: 10.1002/ajh.1107. [DOI] [PubMed] [Google Scholar]
- 51.Hayag-Barin JE, Smith RE, Tucker FC, Jr, et al. Hereditary spherocytosis, thrombocytosis, and chronic pulmonary emboli: a case report and review of the literature. Am J Hematol. 1998;57:82–84. doi: 10.1002/(sici)1096-8652(199801)57:1<82::aid-ajh15>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 52.Aessopos A, Farmakis D, Deftereos S, et al. Cardiovascular effects of splenomegaly and splenectomy in beta-thalas-semia. Ann Hematol. 2005;84:353–357. doi: 10.1007/s00277-004-1002-4. [DOI] [PubMed] [Google Scholar]
- 53.Vichinsky EP. Pulmonary hypertension in sickle cell disease. N Engl J Med. 2004;350:857–859. doi: 10.1056/NEJMp038250. [DOI] [PubMed] [Google Scholar]
- 54.Kietthubthew S, Kisanuki A, Asada Y, Marutsuka K, Funahara Y, Sumiyoshi A. Pulmonary microthromboembolism by injection of sonicated autologous blood in rabbits with splenic artery ligations. Southeast Asian J Trop Med Public Health. 1997;28 Suppl 3:138–140. [PubMed] [Google Scholar]
- 55.Westerman M, et al. Plasma ‘free’ HB is related to red cell derived vesicle numbers in sickle cell anemia and thalassemia intermedia: implications for nitric oxide (NO) scavenging and pulmonary hypertension. Blood. 2004;104(11):465a. [Google Scholar]
- 56.Carnelli V, D'Angelo E, Pecchiari M, Ligorio M. Pulmonary dysfunction in transfusion-dependent patients with thalassemia major. Am J Respir Crit Care Med. 2003;168:180–184. doi: 10.1164/rccm.200211-1292OC. [DOI] [PubMed] [Google Scholar]
- 57.Tai DY, Wang YT, Lou J, Wang WY, Mak KH, Cheng HK. Lungs in thalassaemia major patients receiving regular transfusion. Eur Respir J. 1996;9:1389–1394. doi: 10.1183/09031936.96.09071389. [DOI] [PubMed] [Google Scholar]
- 58.Powars D, Weidman JA, Odom-Maryon T, Niland JC, Johnson C. Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. Medicine (Baltimore) 1988;67:66–76. [PubMed] [Google Scholar]
- 59.De Castro LM, Jonassaint JC, Graham FL, et al. Pulmonary hypertension in SS, SC and Sbeta thalassemia: prevalence, associated clinical sydromes, and mortality [abstract] Blood. 2004;104:462a. [Google Scholar]
- 60.Machado RF, Martyr S, Kato GJ, et al. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. Br J Haematol. 2005;130:445–453. doi: 10.1111/j.1365-2141.2005.05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Machado RF, Gladwin MT. Chronic sickle cell lung disease: new insights into the diagnosis, pathogenesis and treatment of pulmonary hypertension. Br J Haematol. 2005;129:449–464. doi: 10.1111/j.1365-2141.2005.05432.x. [DOI] [PubMed] [Google Scholar]
- 62.Lin EE, Rodgers GP, Gladwin MT. Hemolytic anemia-associated pulmonary hypertension in sickle cell disease. Curr Hematol Rep. 2005;4:117–125. [PubMed] [Google Scholar]
- 63.Lin EE, Gladwin MT, Machado RF. Pulmonary hypertension in patients with hemoglobinopathies: could a mechanism for dysfunction provide an avenue for novel therapeutics? Haematologica. 2005;90:441–444. [PubMed] [Google Scholar]