Abstract
Impulse control disorders (ICDs), including pathological gambling, trichotillomania, kleptomania and others, have been conceptualized to lie along an impulsive-compulsive spectrum. Recent data have suggested that these disorders may be considered addictions. Here we review the genetic and neuropathological bases of the impulse control disorders and consider the disorders within these non-mutually exclusive frameworks.
Introduction
Impulse Control Disorders
Formal impulse control disorders (ICDs) for which there are diagnostic criteria in the Diagnostic and Statistical Manual (DSM-IV-TR) include pathological gambling (PG), kleptomania, pyromania, intermittent explosive disorder, trichotillomania and ICD not otherwise specified [1]. Criteria for other ICDs (compulsive shopping, problematic internet use, compulsive sexual behavior, and compulsive skin picking) have been proposed and are currently under consideration [2, 3]. Basic characteristics of ICDs include repetitive or compulsive engagement in a specific behavior (e.g., gambling, hair-pulling) despite adverse consequences, diminished control over the problematic behavior, and tension or an appetitive urge state prior to engagement in the behavior [2].
ICDs and Addiction
ICDs have been hypothesized to lie along an impulsive-compulsive spectrum [4], representing obsessive-compulsive (OC) spectrum disorders [5, 6]. Although individuals with ICDs engage in repetitive behaviors, often with strong associated urges, behaviors are often related as pleasurable or egosyntonic, whereas repetitive behaviors or rituals in OC disorder (OCD) are generally egodystonic [7, 8]. Individuals with ICDs typically score high on measures of impulsivity and related constructs like sensation-seeking whereas individuals with OCD typically score high on measures of harm avoidance [8-12]. Diagnostic criteria for ICDs like PG overlap with those for substance dependence, with specific criteria relating to tolerance, withdrawal, repeated unsuccessful attempts to cut back or quit, and interference in major areas of life functioning [1]. As outlined below, there are multiple neurobiological and genetic similarities between ICDs and substance addictions. Thus, ICDs may be considered “behavioral addictions” [13-16].
Addiction: An Overview
Extensive research has been performed into the neurobiological underpinnings of the development and maintenance of addictions (reviewed in [17-19]). Emerging views of addiction involve a drug or behavior acquiring saliency via reinforcement, with subsequent transitions through reward-based learning processes into habitual/compulsive levels of engagement [19].
Appetitive conditioning is an important consideration in the early stages of the addiction process. Appetitive conditioning, defined as “the process through which new rewards are learned and acquire their motivational salience,” includes conditioned environmental stimuli that are closely associated in time with addictive processes [20]. Several neuroanatomical structures important in this conditioning process include the amygdala, which is important in the assignment of emotional significance and learned associations between motivationally relevant and otherwise neutral stimuli [17, 21], the orbitofrontal cortex (OFC), which in animal studies has been suggested to encode outcome expectancies and via its strong anatomical connections with the basolateral amygdala (BLA) may facilitate associative learning in the amygdala, and the anterior cingulate cortex (ACC) which has been implicated in discriminative learning and cognitive control [22]. Additional structures that are important in this process include the hippocampus, which provides contextual memory relevant to motivational stimuli, and hypothalamic and septal nuclei, which provide information relevant to primitive motivational behaviors such as sexual drives and nutrient ingestion [23, 24]. Together, these and related structures comprise neurocircuitry that underlies the engagement in motivated behaviors. As motivated behaviors become increasingly subordinated to the addiction-related ones during the progression of the addictive process, it is likely that changes in the structure and function of these regions contribute to the excessive engagement in behaviors that is central to ICDs.
Also important in conditioning and addiction is the nucleus accumbens (NAcc), which is comprised of a shell and a core. The shell, via reciprocal innervation with the ventral tegmental area, is important in modulating motivational salience, whereas the core is more involved with expression of learned behaviors in response to stimuli that predict motivationally relevant events/conditioned reinforcement [17, 19]. The ventral tegmental area (VTA), with its dopaminergic projections to the amygdala, NAcc and prefrontal cortex (PFC, which includes the OFC and ACC), facilitates learned associations with motivationally salient events via phasic dopamine (DA) release [25, 26]. Dopaminergic neurons are inhibited, likely via the dorsal medial thalamus (habenula), when expected rewards do not occur [27, 28]. It has been proposed that in the latter stages of addiction, predominant influence over behavioral drive transitions from corticostriatal circuits that involve the ventral striatum to circuits that involve the dorsal striatum, which has long been implicated in habit formation (see below) [29, 30].
Using the striatum as a focus, a model can be generated in which appetitive conditioning begins in the NAcc shell via inputs from the hippocampus, VTA (which also receives input from the central nucleus of the amygdala), and PFC, “transitions” to conditioned reinforcement in the NAcc core via inputs from the BLA and PFC, and finally evolves to habit formation in the dorsal striatum via input from the sensorimotor cortices and other regions like the septal hypothalamus [19, 23]. These transitions involve limbic, associative and sensorimotor regions of the striatum, respectively (see figure 1A). The dorsal striatum and globus pallidus (via input from the NAcc core) act on the thalamus which then feeds back to cortical structures. Within this anatomical framework, the genetics and neurobiology of ICDs are reviewed. Additionally, though there is much overlap in neurocircuitry and neurotransmitter involvement in different stages of addiction, these systems are presented in an order roughly paralleling the above-mentioned transitional formation of addiction.
Figure 1.
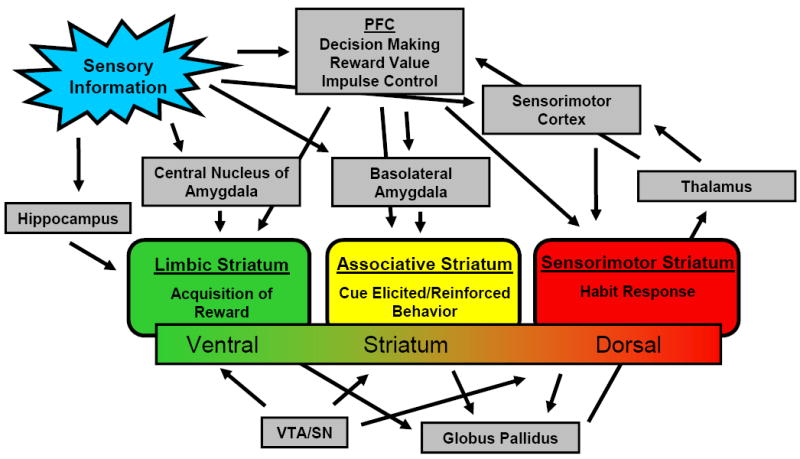
a: Brain circuitry implicated in addiction. PFC = prefrontal cortex, VTA = ventral tegmental area, SN = substantia nigra, NAcc = nucleus accumbens, OFC = orbitofrontal cortex
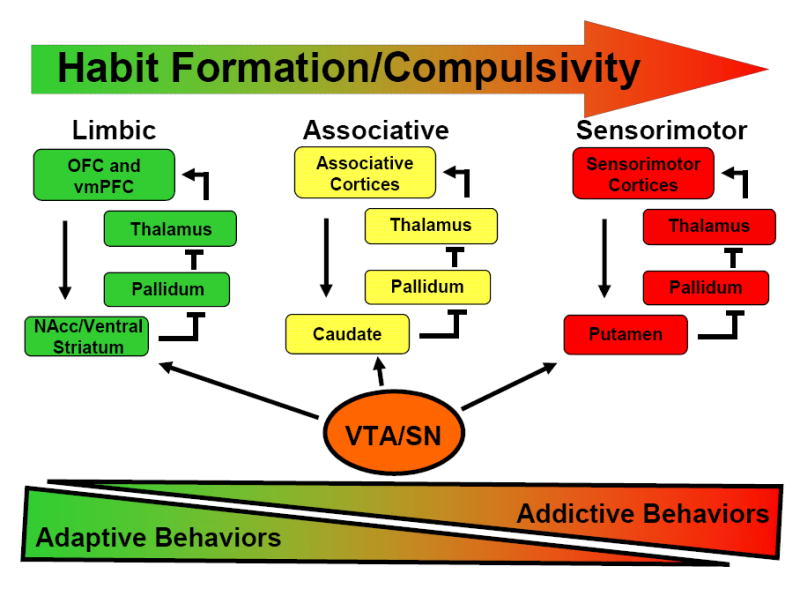
b. Transition of activation of different corticostriatal networks with the formation of habit. Different colors reflect distinct corticostriatal circuits that underlie motivated and habitual behavior. The multicolored arrow represents the shift in involvement of these circuits as behavior becomes more habitual or compulsive in nature. Bottom triangles reflect hypothesized proportions of the motivational behavior repertoire that are devoted to adaptive and addictive behaviors respectively, as involvement of more dorsal striatal circuits become more prominent and addictive behaviors become more severe.
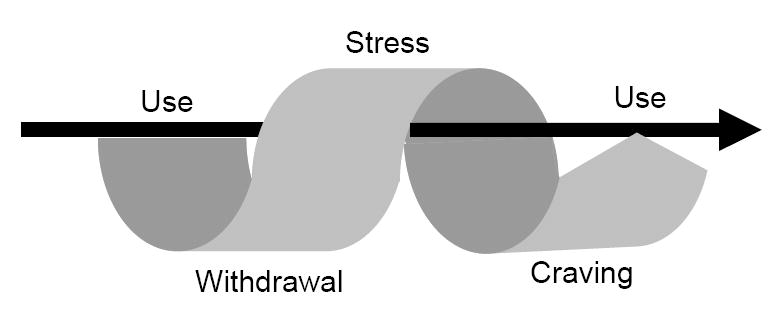
c. Habit formation in Addiction. Habit formation in addiction progression with cyclical arrow representing pattern of behaviors posited as central to the addictive process [178]. Straight arrow represents transition from acquisition to habitual/compulsive behaviors in addiction formation.
Population Genetics of Addiction and ICDs
Genes in essence provide the first contribution to the addiction process, as they determine foundational vulnerabilities for normal behavioral processes to go awry. Genetic studies of ICDs suggest similarities to other addictions [31]. Family and twin epidemiologic studies have estimated that genetic contributions account for up to 60% of the variance in the risk for substance addictions [32, 33]. Similarly robust genetic contributions have been found for PG. Using data from the Vietnam Era Twin (VET) registry, genetic factors were estimated to account for between 35% and 54% of the liability for DSM-III-R symptomatology in PG [34]. The degree of heritability is similar to those of other psychiatric disorders including substance use disorders: in the same sample, 34% of variance in the risk for drug dependence was attributable to genetic factors [35]. Another study of the VET registry assessed lifetime histories of PG and alcohol dependence by structured interview and quantified the extent to which environmental and genetic risk for PG was shared with alcohol dependence. The authors found that a significant proportion of the risk for subclinical PG (12-20% of genetic and 3-8% of environmental) was accounted for by the risk of alcohol dependence [36]. In a subsequent study of the same population, Slutske and colleagues also found a significant association between PG and antisocial behavior, with this association predominantly being explained by genetic factors [37]. These studies suggest that ICDs such as PG are related to alcohol dependence and antisocial behavior, and may be linked via common underlying pathways such as impulsivity (see below). Although preliminary, these data suggest that as with drug addictions, genetic factors contribute significantly to the pathophysiology of ICDs. Specific genetic contributions related to the neurotransmitters implicated in ICDs are described below.
Impulsivity
Impulsivity has relevance for many psychiatric disorders, including ICDs and substance addictions [38]. Within the addiction process, impulsivity contributes to early stages such as drug experimentation. Trait impulsivity has multiple components; e.g., one study identified four components (urgency, lack of premeditation, lack of perseverance, and sensation seeking [39]) whereas other structured measures of impulsiveness factor into three elements (the Barratt Impulsivity Scale fractionates into cognition, motor and planning components and the Eysenck impulsivity scale into venturesomeness, impulsiveness and empathy domains [40, 41]). Moeller and colleagues have defined impulsivity as “a predisposition toward rapid, unplanned reactions to internal or external stimuli [with diminished] regard to the negative consequences of these reaction to the impulsive individual or to others [42].” Together, these findings suggest impulsivity is a complex, multifaceted construct. Consistently, data from human and animal studies suggest that multiple brain regions and neurotransmitter systems contribute to impulsive behaviors throughout the addiction process [32, 43].
Dopamine, Impulsivity and ICDs
As outlined above, dopamine is relevant early in the addiction process as well as in later aspects. Dopaminergic systems have been implicated in impulsivity and ICDs. Psychostimulants such as amphetamine influence dopamine and other biogenic systems and are effective therapies for attention deficit hyperactivity disorder (ADHD), a disorder that has impulsivity as a central feature. Dysregulation of the NAcc DA system has been implicated in ADHD [44]. Dopaminergic systems also contribute to addictive processes. Persistently low D2 receptor availability has also been reported in cocaine abusers several months after detoxification, and this availability has been associated with decreased metabolism in the OFC among other brain regions such as the cingulate gyrus [18, 45]. Low baseline measures of striatal DA D2 receptor availability in non-addicted subjects predict methylphenidate drug liking, supporting the hypothesis that low D2 receptor availability mediates vulnerability to addiction [46]. In support, reduced D2 receptor availability (likely due to decreased receptor numbers rather than increased DA release) was observed in the ventral striatum of highly impulsive rats, and this availability predicted high rates of intravenous cocaine self-administration [47]. Low D2 receptor availability in the striatum also predicted subsequent increased cocaine self-administration by monkeys [48]. The extent to which these findings related to impulsivity and ICDs requires direct examination.
DA may mediate rewarding or reinforcing aspects of gambling, and DA has been implicated in PG [49]. Decreased levels of DA and increased levels of it metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) have been found in the CSF of pathological gamblers [50], although these findings were no longer observed when correcting for CSF flow rate [51]. Amphetamine, a drug that increases extracellular catecholamine and 5-HT concentrations via vesicular depletion, reuptake inhibition, enhancement of DA synthesis, and monoamine oxidase (MAO) inhibition [52], cross-primes for gambling behavior in problem gamblers, but not for alcohol use in problem drinkers [53]. These findings suggest a role for DA (and/or other aminergic pathways) in the pathophysiology of PG as drugs with similar mechanisms of action can cross-prime for reinstatement of other drugs within that class (i.e. amphetamine for cocaine) [54, 55] .
Several reports have linked DA agonist use in Parkinson’s Disease (PD) with PG and other ICD behaviors such as in the domains of sex and eating [56-60]. A recent study of 272 PD patients who were screened and assessed for ICDs found similarly strong associations across DA agonists with PG and other ICDs [61]. A history of an ICD prior to PD onset was associated with a current ICD. Daily levo-dopa equivalence doses were higher in patients with an ICD than in those without. A prospective study of 297 patients with PD screened for lifetime prevalence of PG also found an association between DA agonist use and PG [62]. Although no association was observed with agonist subtype, an association with concurrent levodopa administration was observed, suggesting a total dose effect or priming effect of levodopa [62]. As such, existing data suggest that DA agonists, particularly in individuals at risk for ICDs, are associated with PG and other ICDs, further linking the DA system with ICDs.
Genetic studies have linked several genes to impulsivity and addiction, including genes encoding the DA D4 receptor (DRD4) and DA transporter (SLC6A3) [32, 63, 64] ADHD is highly heritable, with a genetic contributions accounting for nearly 80% of the risk for the disorder, and amongst the most implicated genetic variants related to ADHD are DRD4 and the SLC6A3 variants [65]. Other DA genes such as DRD5 have been linked to ADHD as well [65]. Two studies found an association of polymorphisms of DRD4 with PG [66, 67] . Additionally, the D2A1 allele of the D2 receptor has been implicated in drug abuse, compulsive eating and smoking [63, 68], and has been found in two-fold higher frequency in subjects with PG compared to controls [69]. The above data suggest, both through genetic predispositions and functional output, dopaminergic contributions to impulsive components of ICDs and other addictions. However, additional studies are needed to replicate and extend these findings, particularly as studies investigating DA contributions to personality measures of impulsivity or theoretically related constructs such as novelty seeking have shown varying results in their relationship to DA gene variants [70].
Dopaminergic Regulation and ICDs: Roles for γ-aminobutyric acid (GABA) and Glutamate
γ-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the brain. It is synthesized in nerve terminals from glutamate by the enzyme glutamate decarboxylase. There is evidence of anatomic and functional connectivity between GABA and dopaminergic systems as well as increasing support for effects of modulation of GABAergic systems on substance use disorders [71]. For example, tiagabine, a GABA reuptake inhibitor used primarily to treat seizures, has shown preliminary efficacy in cocaine addiction [72], and has in a case report, shown to help with control of impulsive aggression [73]. Glutamate, an excitatory neurotransmitter and precursor of GABA has also been implicated in addictions as well as ICDs.
In preclinical studies, levels of glutamate within the NAcc mediate reward-seeking behavior [74]. Nonvesicular glutamate release from cysteine/glutamate antiporters has been shown to be the main source of extracellular glutamate in the NAcc; it modulates the release of vesicular glutamate and dopamine via stimulation of glutamate group 2/3 metabotropic glutamate receptors [75, 76]. N-acetylcysteine (NAC), a cysteine pro-drug, increases extracellular levels of glutamate, perhaps via stimulation of inhibitory metabotropic glutamate receptors, thus reducing synaptic release of glutamate. It has shown preliminary efficacy in both cocaine addiction [77] and PG [78]. Taken together, these data suggest possible roles for glutamatergic and GABAergic systems in substance and behavioral addictions.
Serotonin, Impulsivity and ICDs
Like DA, GABA and glutamate, a role for serotonin (5-HT) is supported in impulsivity, ICDs and drug addictions. Serotonergic neurons project form the dorsal raphe nucleus throughout the brain to regions including the hippocampus, frontal cortex and amygdala. In animal models, forebrain 5-HT depletion has been shown to lead to impulsive choice, while the indirect 5-HT agonist fenfluramine decreases such behavior [79, 80]. Additionally, lesion of the rat raphe results in transient preference for immediate rewards [81]. Relatively nonselective 5-HT antagonists have been shown to promote self-controlled choice [82]. A role for specific serotonin system components is supported by findings of greater motor impulsivity in 5-HT1B knock-out mice [83]. Tryptophan depletion, which lowers 5-HT levels (with concomitant decreases in 5-HT metabolites in the cerebrospinal fluid (CSF)), increases motor impulsivity (continuous-performance test-identical pairs), but not impulsive choice (delay discounting) in humans [84, 85]. In subjects with a family history of alcoholism, tryptophan depletion decreases behavioral inhibition (Stop Task) but did not influence delay discounting [84]. Low levels of the 5-HT metabolite 5-hydroxyindolacetic acid (5-HIAA) have been found in individuals with impulsive characteristics [86, 87], and early-onset alcoholism [64]. Low levels of CSF 5-HIAA have also been associated with risk-taking behaviors in primates; e.g., monkeys taking longer leaps in the jungle [88]. Taken together, multiple lines of evidence support a role for 5-HT in mediating impulsivity, though more research is needed to identify the specific 5-HT system components contributing to specific aspects of impulsivity.
5-HT systems have been implicated in ICDs. Although men with PG versus those without show no significant differences in 5-HT or 5-HIAA in CSF samples [50, 89, 90], levels of 5-HIAA were found to be lower in those with PG when controlling for tapping time (which was increased in the PG group) [51]. Metachlorophenylpiperazine (m-CPP), a metabolite of trazodone acts as a partial agonist and has high affinity for 5-HT receptors (especially 5-HT2c, which has been implicated in the mediation of aspects of mood, anxiety behavior and neuroendocrine function [91]). Administration of m-CPP has been reported to generate a behavioral “high” and increase prolactin levels (a process thought to be mediated by postsynaptic 5-HT1A/2A/2C receptors) in subjects with PG as compared to control subjects without PG [92]. This subjective response is similar to that reported in other disorders in which impulsive or compulsive behaviors are prominent, including antisocial personality disorder [93], borderline personality disorder [94], cocaine dependence [95], and alcohol abuse or dependence [96].
In addition to pharmacological challenges, genetic studies have implicated the 5-HT system in both impulsivity and ICDs. A TPH1 (tryptophan hydroxylase 1, which encodes the enzyme for the rate-limiting step in 5-HT production) gene variant has been found to be associated with reduced 5-HIAA in CSF and suicidal behavior in impulsive violent criminal offenders [97]. Other serotonergic genes have been linked to both impulsivity and substance addiction and include SERT (SLC6A4) and MAOA [32]. A polymorphism in the promoter region of the human serotonin transporter gene (SLC6A4) encoding short and long forms of the protein (with the short variant producing functionally less protein) has been associated with several dimensions of psychopathology, including neuroticism, anxiety and depression [98-102], though more recent studies have raised questions regarding the strength or validity of these associations [103-105]. SLC6A4 variation may contribute to ICDs as an association has been reported between the SLC6A4 short allele and PG in males but not females [106]. Finally, studies involving small samples of subjects have inconsistently reported links between serotonin and monoamine oxidase genes and ICDs such as PG, compulsive buying and trichotillomania [107-109]. Additional studies using larger samples and careful (e.g., diagnostic) assessments will help to investigate the genetics of the broader family of ICDs.
Treatment studies of serotonergic agents have yielded mixed results regarding efficacy in treatment of ICDs [110-113]. Placebo-controlled, randomized clinical trials (RCTs) of selective serotonin reuptake inhibitors (SSRIs) have yielded mixed results, with some RCTs showing superior efficacy over placebo [114, 115] and others not [116, 117] . Most studies showed clinical improvement early in treatment in both drug- and placebo-treated groups. These gains suggest a treatment or placebo response rather than gains specific to the active drug, although later differentiation between groups in some studies suggest active medication effects. In several studies of trichotillomania, no significant difference was observed between fluoxetine and placebo treatments [111]. In a randomized study of citalopram vs. placebo in 28 homosexual men with compulsive sexual behaviors, no differences were seen in measures of compulsive sexual behaviors between groups after 12 weeks of therapy, although there was a significant decrease in sexual drive related to active drug [118]. Two parallel-arm, controlled studies of fluvoxamine in the treatment of compulsive buying showed no difference between active drug and placebo [119, 120], but a study of seven weeks of open-label citalopram followed by nine weeks of randomization showed improvement in active drug compared to placebo [121]. A case report suggested efficacy of escitalopram, and SSRI in the treatment of problematic internet use, but further studies need to be conducted regarding efficacy in treatment (and diagnosis) of this disorder [113]. Taken together, the findings suggest that SSRIs work for some individuals with ICDs but not others. These findings suggest that specific individual features (e.g., genetic features or co-occurring disorders like anxiety or depression) might help guide the selection of appropriate treatments [122].
As described above, impulsivity contributes to both ICDs and substance addictions. It is likely that impulsivity has unique contributions to individual ICDs and substance addictions as is the case for aspects of cognitive functioning [123]. Additionally, like with impulsivity, similarities between ICDs and substance addictions exist in other domains, such as decision-making and stress responsivity, and these domains are considered below.
Risk-Reward Assessment, Decision-making, and Ventral Prefrontal Cortex (PFC)
Once a behavior has moved beyond the initial stages of associative learning, executive control over its execution becomes increasingly important. Regions of the PFC contribute to decision-making in disorders of impulse control and addiction. The OFC codes the relative value of reward stimuli [124, 125], a process in part mediated by the 5-HT system. The OFC facilitates cognitive flexibility by promoting updating of associative encoding in downstream brain areas such as the amygdala [126]. Additionally, the inferior frontal gyrus/dorsolateral PFC is important in shifting attention, which contributes to the ability to resist intrusive information such as thinking about drugs/behaviors [127]. The OFC, including the overlapping ventromedial PFC (vmPFC), contributes to reward processing and prediction [128, 129] . Subjects with vmPFC lesions show characteristic deficits in planning, often repeatedly making decisions that lead to negative consequences [130]. Further, these subjects also perform worse than control comparison subjects on the Iowa Gambling Task (IGT), a measure which was developed to investigate small immediate reward and intermittent punishment associated with long-term gain versus large immediate reward and intermittent punishment associated with long-term loss [131].
Subjects with substance use disorders typically display impaired performance on the IGT [132], and this poor performance has been correlated with decreased blood flow to the vmPFC and other cortical regions [133-136]. Individuals with PG also choose disadvantageously as compared to controls on the IGT [12, 137]. Individuals with PG more readily choose lower monetary rewards promised immediately over higher monetary rewards promised after delayed intervals (“delay discounting”) compared to control subjects [138]. Temporal discounting of rewards has been shown to be more rapid in individuals with PG with comorbid substance use disorders, consistent with mechanisms contributing to each disorder in an additive or synergistic fashion [138]. Dysfunction of vmPFC circuitry may contribute to these differences in behaviors between PG and control subjects, as appears to be the case in drug addiction. Decreased activation of the vmPFC has been observed in PG subjects during presentation of gambling cues [9], performance of the Stroop Color-Word Interference Task [139], and simulated gambling [140]. In this last study, activation of the vmPFC correlated inversely with gambling severity among PG subjects. Together, these data suggest an important role for the vmPFC in PG. Future studies will help to elucidate the extent to which these finding extend to other ICDs.
Substance-dependent individuals show abnormalities in the OFC. Similar to individuals with damage to the OFC, subjects with stimulant dependence show sub-optimal decision making, with longer deliberation before choice selection [141]. Diminished activation of the OFC and cingulate gyrus has been associated with chronic cocaine use [142]. Poor performance on a color-word drug Stroop task correlates with hypoactivation of the OFC in cocaine-addicted individuals [142]. Taken together, these data suggest regions of the PFC are important in decision-making.
Decision-Making, Impulsivity, and the Amygdala
Amygdala function contributes significantly to decision-making and impulsivity. The amygdala receives serotonergic and dopaminergic input from the raphe and VTA respectively, and its activation is regulated by a balance between glutamate-induced excitation and GABA-mediated inhibition [143, 144]. The amygdala participates in the processing and memory of emotional reactions. According to the somatic marker hypothesis (which states that decision making relies on neural substrates that regulate homeostasis, feeling and emotion), affective responses to stimuli are evoked through visceral motor structures such as the hypothalamus and other autonomic brainstem nuclei [127]. The amygdala works in conjunction with the vmPFC/OFC in decision-making, with each region contributing in a distinct fashion. In rodents, excitotoxic lesions of the BLA promote impulsive choice in a delayed-reinforcement task [145]. In humans, subjects with vmPFC damage and subjects with amygdalar damage both demonstrate deficiencies in decision-making in the IGT [146]. However, autonomic responses (measured by skin conductance response) to large monetary gains or losses are deficient in individuals with bilateral amygdalar lesions; in contrast, these responses are intact in patients with vmPFC damage [146]. However, anticipatory skin conductance responses during IGT performance show a different pattern: subjects with vmPFC damage show deficiencies, whereas those with amygdalar damage show normal responses. Together, these findings that abnormal amygdala-ventral striatum activity may influence impulsivity in addictive processes, possibly through an effect on incentive value attribution of cues [148]. In drug-addicted persons, exaggerated autonomic responses are triggered by drug cues [149]. Abnormal amygdalar activity can be influenced by genetic variants in 5-HT genes [100]. The role of the amygdala in ICDs has not been directly investigated.
Habit Formation
As a behavior shifts from active learning to habitual response, control shifts from an associative cortico-basal ganglia network involving the PFC and ventral striatum to dorsomedial striatum/caudate and then to a more sensorimotor cortico-basal ganglia network involving the dorsolateral striatum/putamen (see Figure 1b) [29]. Overtraining of behaviors shifts activation from dorsolateral PFC and caudate to putamen and motor cortices [150, 151]. In addiction, repeated cocaine self-administration in monkeys is related to a progression of activation of the ventral striatum to involvement of the dorsal striatum [152]. As behavior becomes habitual, conditioned stimuli, important components of the addictive process, tend to potentiate habit responses rather than goal-directed activity [153]. This differential response may be influenced indirectly by the NAcc via its projections to the VTA/substantia nigra with subsequent dopaminergic input from the latter to the sensorimotor network [154]. Infusion of the mixed DA receptor antagonist alpha-flupenthixol in the dorsal striatum but not into the NAcc core reduces established cocaine seeking in animal models of addiction [155]. Down-regulation of D2 DA receptors has been observed first in ventral and then dorsal striatum in cocaine-taking monkeys, consistent with observations made with human chronic cocaine abusers [156, 157].
ICDs have been described in terms of habit formation [158]. As with drug addictions, dysregulation of striatal circuitry is implicated in these disorders. For example, in a study of simulated gambling, individuals with PG showed differences in striatal activation as compared with control subjects, and activation was related to gambling severity [140]. Preliminary data similarly implicate striatal function in gambling urges in PG and in cocaine cravings in cocaine dependence [159]. Relatively diminished putamenal volume has been observed in subjects with trichotillomania as compared to control subjects, although the functional relevance of this anatomical difference requires additional investigation [160]. From these data, a hypothesis can be constructed that goal-directed actions transit from active learning to a more dysfunctional, habit-based response in ICDs in a similar fashion to that observed in substance-addicted individuals.
Stress Responsiveness and ICDs
Stressful events and psychological distress frequently contribute to relapse to drug use among individuals with opiate and cocaine dependence [161, 162]. Preclinical evidence indicates that acute stress leads to increases in self-administration of drugs such as amphetamines [163], cocaine [164, 165], and alcohol [166, 167]. Mechanisms related to stress are critical in the establishment of addictions and their propagation as chronic disorders [168]. Stress exposure produces an increased arousal state similar to drugs themselves [169]. A number of drugs of abuse, such as psychostimulants [170-172] and alcohol [173] activate stress circuitry and the HPA axis. In rodents, opioids stimulate the HPA axis, but the opposite effect is seen in primates, including humans (reviewed in [174]). Additionally, benzodiazepines have been shown to attenuate HPA activation in humans [175] As activation of the HPA axis reciprocally increases mesolimbic dopamine transmission, exposure to stress may provide a common neural substrate by which stress enhances drug-seeking behavior [169]. Stress-related stimuli, such as restraint and footshock, increase NAcc DA release [176, 177]. Stress-induced craving paradigms in treatment-engaged addicted individuals activate the striatum and decrease activation in the anterior cingulate. These findings suggest a role for stress in prefrontal dysfunction and concurrent engagement of habit circuitry in addiction [178]. The extent to which these changes are related to impulsivity and/or disadvantageous decision-making requires further investigation [179].
Studies of individuals with ICDs have yielded varying results regarding the involvement of stress pathways in these disorders [180]. For example, CSF levels of corticotrophin-releasing hormone (CRH) did not differ in subjects with PG compared to controls [89]. Transient increases in cortisol have been noted in gambling studies of volunteers recruited from casinos with problem gamblers showing a similar magnitude of response to controls [181-183]. Stressful life events like early life trauma have been implicated in PG like they have in drug addiction [177]. Together, these data suggest that it will be important to examine further the precise mechanisms in which stress and stress pathways contribute to the pathophysiology of ICDs.
Opioids, Stress and ICDs
Opioids modulate mesolimbic DA pathways in the VTA by activating μ opioid receptors on secondary interneurons causing hyperpolarization and inhibition of GABA release on primary neurons (the dopaminergic output neurons) with consequent increased DA release [184]. However, activation of κ opioid receptors on primary neurons causes their direct inhibition [185]. Recently it has been shown that opioid receptor activation (κ vs. μ) differentially inhibits mesolimbic neurons depending on their target projections (Nacc vs. BLA) [186]. The endogenous opioid system, via both μ and κ opioid receptors, tonically inhibits the HPA axis, suggesting that atypical responsivity contributes to addiction [32]. In support of this hypothesis, mice that lack the mu opioid receptor gene (OPRM1) show no morphine analgesia or place preference [187].
Polymorphisms in OPRM1 are associated with differential binding to endorphins (for example, the A118G variant codes for a receptor with three-fold greater binding and activation of its G protein-coupled inwardly rectifying potassium channel [188]). The A118G variant has been associated with opioid dependence [32], and subjects with this variant have shown more favorable responses to naltrexone for treatment of alcohol dependence [64, 189]. Haplotypes of the kappa opioid receptor gene (OPRK1) and the promoter region of its endogenous ligand precursor, prodynorphin, have also been associated with opiate dependence and other addictions [33].
Gambling or related behaviors have been associated with elevated blood levels of the endogenous opioid β-endorphin [190]. Given their mechanism of action [191] and efficacy in the treatment of alcohol and opiate dependence [192], opioid receptor antagonists have been examined in the treatment of ICDs. Naltrexone has shown superiority to placebo in a single-site study of PG [193], and nalmefene, a long-acting opioid antagonist, has shown superiority to placebo in a large double-blind, multi-centered study of subjects with PG [194]. Naltrexone has shown benefit in case studies of compulsive sexual behavior [195] and open-labeled trials of adolescent sexual offenders [196]. Naltrexone has shown preliminary efficacy in compulsive buying [121]. These data suggest that, opioid systems are important in both chemical and behavioral addictions. As opioids influence multiple neural networks and stress-related pathways, future studies will likely define their precise mechanisms of action in ICDs.
Conclusions and Future Directions
Emerging data on the neurobiology of impulsivity and ICDs suggest parallels with drug addictions. Although many fewer studies have investigated ICDs than have drug addictions (and most existing studies have investigated PG), genetic, behavioral and treatment data implicate multiple neurotransmitter systems and neuronal circuits in the establishment and maintenance of behavioral addictions. Despite these advances, controversy remains regarding the nosology and underlying pathophysiology of specific ICDs.
Endophenotypes provide insight into the etiology of disorders and such information can inform the categorizations of disorders. Endophenotypic views of psychiatric disorders like depression and schizophrenia are emerging [197, 198]. Endophenotypes are “measurable components unseen by the unaided eye” and may be neuropsychological, endocrinological, cognitive, neuroanatomical or biochemical in nature. Endophenotypes inform the understanding of genetic factors underlying disease processes by focusing on specific biological features rather than diagnostic categories which in psychiatry are typically heterogeneous in nature [198]. As more becomes known as to the nature and characterization of ICDs, endophenotypic views of their underlying components may emerge. For example, impulsivity, differential endocrine responses to stress, or components thereof may represent important endophenotypes for PG, other ICDs and substance addictions. Identifying endophenotypes should help differentiate subclasses of disorders (genetically based and otherwise), ultimately honing characterization, diagnosis and optimal treatment. Changes in similar endophenotypic measures might be expected to accompany symptom improvement for both ICDs and substance addictions. Clinically relevant endophenotypes may also guide the development of animal models of these diseases that will ultimately help us understand the etiology of ICDs and substance addictions, develop more effective prevention strategies and optimize behavioral and pharmacological treatments.
Acknowledgments
We would like to thank Dr. Christopher Pittenger for his thorough review of and helpful comments regarding this manuscript. Support for this research was provided by NIH grant T32-MH19961 Clinical Neuroscience Research Training in Psychiatry (JAB), a Mind and Life Institute Research Varela Grant (JAB), the National Institute on Drug Abuse grants R01-DA019039 (MNP) and R01-DA020908 (MNP), Women’s Health Research at Yale (MNP), and the VA VISN1 MIRECC (MNP) and REAP (MNP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association Committee on Nomenclature and Statistics. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Grant J, Potenza MN. Impulse control disorders: clinical characteristics and pharmacological management. Ann Clin Psychiatry. 2004;16:27–34. doi: 10.1080/10401230490281366. [DOI] [PubMed] [Google Scholar]
- 3.Grant JE, Potenza MN. Compulsive aspects of impulse-control disorders. The Psychiatric clinics of North America. 2006;29(2):539–51. x. doi: 10.1016/j.psc.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McElroy SL, Hudson JI, Pope H, Jr, Keck PE, Jr, Aizley HG. The DSM-III-R impulse control disorders not elsewhere classified: clinical characteristics and relationship to other psychiatric disorders. Am J Psychiatry. 1992;149(3):318–27. doi: 10.1176/ajp.149.3.318. [DOI] [PubMed] [Google Scholar]
- 5.Hollander E, Wong CM. Obsessive-compulsive spectrum disorders. J Clin Psychiatry. 1995;56(Suppl 4):3–6. discussion 53-5. [PubMed] [Google Scholar]
- 6.Hollander E, Wong CM. Body dysmorphic disorder, pathological gambling, and sexual compulsions. J Clin Psychiatry. 1995;56(Suppl 4):7–12. discussion 13. [PubMed] [Google Scholar]
- 7.Grant J, Potenza MN. Compulsive aspects of impulse control disorders. Psychiatr Clin N Am. doi: 10.1016/j.psc.2006.02.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaszczynski A. Pathological gambling and obsessive-compulsive spectrum disorders. Psychol Rep. 1999;84(1):107–13. doi: 10.2466/pr0.1999.84.1.107. [DOI] [PubMed] [Google Scholar]
- 9.Potenza MN, Steinberg MA, Skudlarski P, Fulbright RK, Lacadie CM, Wilber MK, et al. Gambling urges in pathological gambling: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60(8):828–36. doi: 10.1001/archpsyc.60.8.828. [DOI] [PubMed] [Google Scholar]
- 10.Won Kim S, Grant JE. Personality dimensions in pathological gambling disorder and obsessive-compulsive disorder. Psychiatry Research. 2001;104(3):205–212. doi: 10.1016/s0165-1781(01)00327-4. [DOI] [PubMed] [Google Scholar]
- 11.Petry NM. Psychiatric symptoms in problem gambling and non-problem gambling substance abusers. American Journal on Addictions. 2000;9(2):163–171. doi: 10.1080/10550490050173235. [DOI] [PubMed] [Google Scholar]
- 12.Cavedini P, Riboldi G, Keller R, Annucci A, Bellodi L. Frontal lobe dysfunction in pathological gambling patients. Biological Psychiatry. 2002;51(4):334–341. doi: 10.1016/s0006-3223(01)01227-6. [DOI] [PubMed] [Google Scholar]
- 13.Potenza M. Should Addictive Disorders Include Non-Substance-Related Conditions? Addiction. 2006;101(suppl 1):142–51. doi: 10.1111/j.1360-0443.2006.01591.x. [DOI] [PubMed] [Google Scholar]
- 14.Shaffer HJ. Strange bedfellows: a critical view of pathological gambling and addiction. Addiction. 1999;94(10):1445–8. doi: 10.1046/j.1360-0443.1999.941014451.x. [DOI] [PubMed] [Google Scholar]
- 15.Holden C. ‘Behavioral’ addictions: do they exist? Science. 2001;294(5544):980–2. doi: 10.1126/science.294.5544.980. [DOI] [PubMed] [Google Scholar]
- 16.Grant JE, Brewer JA, Potenza MN. The neurobiology of substance and behavioral addictions. CNS spectrums. 2006;11(12):924–30. doi: 10.1017/s109285290001511x. [DOI] [PubMed] [Google Scholar]
- 17.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 18.Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Soelch C, Linthicum J, Ernst M. Appetitive conditioning: Neural bases and implications for psychopathology. Neuroscience and biobehavioral reviews. 2007;31(3):426–40. doi: 10.1016/j.neubiorev.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Annals of the New York Academy of Sciences. 2003;985:233–50. [PubMed] [Google Scholar]
- 22.Parkinson JA, Cardinal RN, Everitt BJ. Limbic cortical-ventral striatal systems underlying appetitive conditioning. Progress in brain research. 2000;126:263–85. doi: 10.1016/S0079-6123(00)26019-6. [DOI] [PubMed] [Google Scholar]
- 23.Chambers R, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain research. 2000;886(12):113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 25.Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. Journal of neurophysiology. 1994;72(2):1024–7. doi: 10.1152/jn.1994.72.2.1024. [DOI] [PubMed] [Google Scholar]
- 26.Schultz W. Behavioral theories and the neurophysiology of reward. Annual review of psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 27.Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. The Journal of neuroscience. 1986;6(3):613–9. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. The Journal of neuroscience. 2003;23(10):4308–14. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature reviews. 2006;7(6):464–76. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 30.Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends in Molecular Medicine. 2006;12(12):559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Lobo DS, Kennedy JL. The genetics of gambling and behavioral addictions. CNS spectrums. 2006;11(12):931–9. doi: 10.1017/s1092852900015121. [DOI] [PubMed] [Google Scholar]
- 32.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature neuroscience. 2005;8(11):1450–7. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- 33.Kreek MJ, Bart G, Lilly C, LaForge KS, Nielsen DA. Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacological reviews. 2005;57(1):1–26. doi: 10.1124/pr.57.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Eisen SA, Lin N, Lyons MJ, Scherrer JF, Griffith K, True WR, et al. Familial influences on gambling behavior: an analysis of 3359 twin pairs. Addiction. 1998;93(9):1375–84. doi: 10.1046/j.1360-0443.1998.93913758.x. [DOI] [PubMed] [Google Scholar]
- 35.Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, et al. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. Am J Med Genet. 1996;67(5):473–7. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 36.Slutske WS, Eisen S, True WR, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for pathological gambling and alcohol dependence in men. Arch Gen Psychiatry. 2000;57(7):666–73. doi: 10.1001/archpsyc.57.7.666. [DOI] [PubMed] [Google Scholar]
- 37.Slutske WS, Eisen S, Xian H, True WR, Lyons MJ, Goldberg J, et al. A twin study of the association between pathological gambling and antisocial personality disorder. Journal of abnormal psychology. 2001;110(2):297–308. doi: 10.1037//0021-843x.110.2.297. [DOI] [PubMed] [Google Scholar]
- 38.Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146(4):348–61. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 39.Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30(4):669–689. [Google Scholar]
- 40.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of clinical psychology. 1995;51(6):768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 41.Eysenck SB, Eysenck HJ. Impulsiveness and venturesomeness: their position in a dimensional system of personality description. Psychological reports. 1978;43(3 Pt 2):1247–55. doi: 10.2466/pr0.1978.43.3f.1247. [DOI] [PubMed] [Google Scholar]
- 42.Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158(11):1783–93. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- 43.Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Annals of the New York Academy of Sciences. 2004;1021:33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- 44.Sagvolden T, Sergeant JA. Attention deficit/hyperactivity disorder--from brain dysfunctions to behaviour. Behavioural brain research. 1998;94(1):1–10. [PubMed] [Google Scholar]
- 45.Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. 2. Vol. 14. Synapse; New York, NY: 1993. pp. 169–77. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. 2. Vol. 46. Synapse; New York, NY: 2002. pp. 79–82. [DOI] [PubMed] [Google Scholar]
- 47.Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Laane K, et al. Nucleus Accumbens D2/3 Receptors Predict Trait Impulsivity and Cocaine Reinforcement. Science. 2007;315(5816):1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9(8):1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- 49.DeCaria C, Begaz T, Hollander E. Serotonergic and noradrenergic function in pathological gambling. CNS Spectrums. 1998;3(6):38–47. [Google Scholar]
- 50.Bergh C, Eklund T, Sodersten P, Nordin C. Altered dopamine function in pathological gambling. Psychol Med. 1997;27(2):473–5. doi: 10.1017/s0033291796003789. [DOI] [PubMed] [Google Scholar]
- 51.Nordin C, E T. Altered CSF 5-HIAA dospositon in pathologic male gamblers. CNS Spectrums. 1999;4(12):25–33. doi: 10.1017/s1092852900006799. [DOI] [PubMed] [Google Scholar]
- 52.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: A review. Progress in Neurobiology. 2005;75(6):406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Zack M, Poulos CX. Amphetamine primes motivation to gamble and gambling-related semantic networks in problem gamblers. Neuropsychopharmacology. 2004;29(1):195–207. doi: 10.1038/sj.npp.1300333. [DOI] [PubMed] [Google Scholar]
- 54.Shalev U, Grimm JW, Shaham Y. Neurobiology of Relapse to Heroin and Cocaine Seeking: A Review. Pharmacol Rev. 2002;54(1):1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 55.Loba P, Stewart SH, Klein RM, Blackburn JR. Manipulations of the features of standard video lottery terminal (VLT) games: effects in pathological and non-pathological gamblers. J Gambl Stud. 2001;17(4):297–320. doi: 10.1023/a:1013639729908. [DOI] [PubMed] [Google Scholar]
- 56.Weintraub D, Potenza MN. Impulse control disorders in Parkinson’s disease. Current neurology and neuroscience reports. 2006;6(4):302–6. doi: 10.1007/s11910-006-0022-y. [DOI] [PubMed] [Google Scholar]
- 57.Kurlan R. Disabling repetitive behaviors in Parkinson’s disease. Mov Disord. 2004;19(4):433–7. doi: 10.1002/mds.10625. [DOI] [PubMed] [Google Scholar]
- 58.Driver-Dunckley E, Samanta J, Stacy M. Pathological gambling associated with dopamine agonist therapy in Parkinson’s disease. Neurology. 2003;61(3):422–423. doi: 10.1212/01.wnl.0000076478.45005.ec. [DOI] [PubMed] [Google Scholar]
- 59.Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE. Pathological Gambling Caused by Drugs Used to Treat Parkinson Disease. Arch Neurol. 2005;62(9):1377–1381. doi: 10.1001/archneur.62.9.noc50009. [DOI] [PubMed] [Google Scholar]
- 60.Szarfman A, Doraiswamy PM, Tonning JM, Levine JG. Association Between Pathologic Gambling and Parkinsonian Therapy as Detected in the Food and Drug Administration Adverse Event Database. Arch Neurol. 2006;63(2):299a–300. doi: 10.1001/archneur.63.2.299-b. [DOI] [PubMed] [Google Scholar]
- 61.Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Archives of neurology. 2006;63(7):969–73. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Voon V, Hassan K, Zurowski M, Duff-Canning S, de Souza M, Fox S, et al. Prospective prevalence of pathologic gambling and medication association in Parkinson disease. Neurology. 2006;66(11):1750–2. doi: 10.1212/01.wnl.0000218206.20920.4d. [DOI] [PubMed] [Google Scholar]
- 63.Haile CN, Kosten TR, Kosten TA. Genetics of dopamine and its contribution to cocaine addiction. Behavior genetics. 2007;37(1):119–45. doi: 10.1007/s10519-006-9115-2. [DOI] [PubMed] [Google Scholar]
- 64.Kreek MJ, Nielsen DA, LaForge KS. Genes associated with addiction: alcoholism, opiate, and cocaine addiction. Neuromolecular medicine. 2004;5(1):85–108. doi: 10.1385/NMM:5:1:085. [DOI] [PubMed] [Google Scholar]
- 65.Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, et al. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychology review. 2007;17(1):39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- 66.Perez de Castro I, Ibanez A, Torres P, Saiz-Ruiz J, Fernandez-Piqueras J. Genetic association study between pathological gambling and a functional DNA polymorphism at the D4 receptor gene. Pharmacogenetics. 1997;7(5):345–8. [PubMed] [Google Scholar]
- 67.Comings DE, Gonzalez N, Wu S, Gade R, Muhleman D, Saucier G, et al. Studies of the 48 bp repeat polymorphism of the DRD4 gene in impulsive, compulsive, addictive behaviors: Tourette syndrome, ADHD, pathological gambling, and substance abuse. Am J Med Genet. 1999;88(4):358–68. doi: 10.1002/(sici)1096-8628(19990820)88:4<358::aid-ajmg13>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 68.Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Comings DE. Dopamine D2 receptor gene variants: association and linkage studies in impulsive-addictive-compulsive behaviour. Pharmacogenetics. 1995;5(3):121–41. doi: 10.1097/00008571-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 69.Comings DE, Rosenthal RJ, Lesieur HR, Rugle LJ, Muhleman D, Chiu C, et al. A study of the dopamine D2 receptor gene in pathological gambling. Pharmacogenetics. 1996;6(3):223–34. doi: 10.1097/00008571-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Gelernter J, Kranzler H, Coccaro E, Siever L, New A, Mulgrew CL. D4 dopamine-receptor (DRD4) alleles and novelty seeking in substance-dependent, personality-disorder, and control subjects. Am J Hum Genet. 1997;61(5):1144–52. doi: 10.1086/301595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sofuoglu M, Kosten TR. Emerging pharmacological strategies in the fight against cocaine addiction. Expert opinion on emerging drugs. 2006;11(1):91–8. doi: 10.1517/14728214.11.1.91. [DOI] [PubMed] [Google Scholar]
- 72.Gonzalez G, Desai R, Sofuoglu M, Poling J, Oliveto A, Gonsai K, et al. Clinical efficacy of gabapentin versus tiagabine for reducing cocaine use among cocaine dependent methadone-treated patients. Drug and alcohol dependence. 2007;87(1):1–9. doi: 10.1016/j.drugalcdep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 73.Kaufman KR, Kugler SL, Sachdeo RC. Tiagabine in the Management of Postencephalitic Epilepsy and Impulse Control Disorder. Epilepsy & Behavior. 2002;3(2):190–194. doi: 10.1006/ebeh.2002.0319. [DOI] [PubMed] [Google Scholar]
- 74.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. The Journal of neuroscience. 2003;23(8):3531–7. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. The Journal of neuroscience. 2002;22(20):9134–41. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. The regulation of dopamine transmission by metabotropic glutamate receptors. The Journal of pharmacology and experimental therapeutics. 1999;289(1):412–6. [PubMed] [Google Scholar]
- 77.Larowe SD, Mardikian P, Malcolm R, Myrick H, Kalivas P, McFarland K, et al. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am J Addict. 2006 Jan-Feb;15(1):105–10. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grant JE, Kim SW, Odlaug BL. N-Acetyl Cysteine, a Glutamate-Modulating Agent, in the Treatment of Pathological Gambling: A Pilot Study. 2007 doi: 10.1016/j.biopsych.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 79.Poulos CX, Parker JL, Le AD. Dexfenfluramine and 8-OH-DPAT modulate impulsivity in a delay-of-reward paradigm: implications for a correspondence with alcohol consumption. 1996;7(4):395–399. doi: 10.1097/00008877-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 80.Mobini S, Chiang TJ, Al-Ruwaitea AS, Ho MY, Bradshaw CM, Szabadi E. Effect of central 5-hydroxytryptamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology. 2000;149(3):313–8. doi: 10.1007/s002130000385. [DOI] [PubMed] [Google Scholar]
- 81.Bizot J, Le Bihan C, Puech AJ, Hamon M, Thiebot M. Serotonin and tolerance to delay of reward in rats. Psychopharmacology. 1999;146(4):400–12. doi: 10.1007/pl00005485. [DOI] [PubMed] [Google Scholar]
- 82.Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128(2):161–70. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- 83.Brunner D, Hen R. Insights into the neurobiology of impulsive behavior from serotonin receptor knockout mice. Annals of the New York Academy of Sciences. 1997;836:81–105. doi: 10.1111/j.1749-6632.1997.tb52356.x. [DOI] [PubMed] [Google Scholar]
- 84.Crean J, Richards JB, de Wit H. Effect of tryptophan depletion on impulsive behavior in men with or without a family history of alcoholism. Behavioural brain research. 2002;136(2):349–57. doi: 10.1016/s0166-4328(02)00132-8. [DOI] [PubMed] [Google Scholar]
- 85.Walderhaug E, Lunde H, Nordvik JE, Landro NI, Refsum H, Magnusson A. Lowering of serotonin by rapid tryptophan depletion increases impulsiveness in normal individuals. Psychopharmacology. 2002;164(4):385–91. doi: 10.1007/s00213-002-1238-4. [DOI] [PubMed] [Google Scholar]
- 86.Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33(26):2609–14. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- 87.Coccaro EF, Siever LJ, Klar HM, Maurer G, Cochrane K, Cooper TB, et al. Serotonergic studies in patients with affective and personality disorders. Correlates with suicidal and impulsive aggressive behavior. Arch Gen Psychiatry. 1989;46(7):587–99. doi: 10.1001/archpsyc.1989.01810070013002. [DOI] [PubMed] [Google Scholar]
- 88.Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, et al. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. The American journal of psychiatry. 1994;151(10):1485–91. doi: 10.1176/ajp.151.10.1485. [DOI] [PubMed] [Google Scholar]
- 89.Roy A, Adinoff B, Roehrich L, Lamparski D, Custer R, Lorenz V, et al. Pathological gambling. A psychobiological study. Arch Gen Psychiatry. 1988;45(4):369–73. doi: 10.1001/archpsyc.1988.01800280085011. [DOI] [PubMed] [Google Scholar]
- 90.Roy A, De Jong J, Linnoila M. Extraversion in pathological gamblers. Correlates with indexes of noradrenergic function. Arch Gen Psychiatry. 1989;46(8):679–81. doi: 10.1001/archpsyc.1989.01810080009001. [DOI] [PubMed] [Google Scholar]
- 91.Kennett GA, Curzon G. Evidence that hypophagia induced by mCPP and TFMPP requires 5-HT1C and 5-HT1B receptors; hypophagia induced by RU 24969 only requires 5-HT1B receptors. Psychopharmacology (Berl) 1988;96(1):93–100. doi: 10.1007/BF02431539. [DOI] [PubMed] [Google Scholar]
- 92.Pallanti S, Bernardi S, Quercioli L, DeCaria C, Hollander E. Serotonin dysfunction in pathological gamblers: increased prolactin response to oral m-CPP versus placebo. CNS spectrums. 2006;11(12):956–64. doi: 10.1017/s1092852900015145. [DOI] [PubMed] [Google Scholar]
- 93.Moss HB, Yao JK, Panzak GL. Serotonergic responsivity and behavioral dimensions in antisocial personality disorder with substance abuse. Biol Psychiatry. 1990;28(4):325–38. doi: 10.1016/0006-3223(90)90660-t. [DOI] [PubMed] [Google Scholar]
- 94.Hollander E, De Caria C, Stein D, Simeon D, Cohen L, Hwang M, et al. Behavioral response to m-CPP. Biol Psychiatry. 1994;35(6):426–7. doi: 10.1016/0006-3223(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 95.Buydens-Branchey L, Branchey M, Fergeson P, Hudson J, McKernin C. The meta-chlorophenylpiperazine challenge test in cocaine addicts: hormonal and psychological responses. Biological psychiatry. 1997;41(11):1071–86. doi: 10.1016/S0006-3223(96)00182-5. [DOI] [PubMed] [Google Scholar]
- 96.Benkelfat C, Murphy DL, Hill JL, George DT, Nutt D, Linnoila M. Ethanollike properties of the serotonergic partial agonist m-chlorophenylpiperazine in chronic alcoholic patients. Arch Gen Psychiatry. 1991;48(4):383. doi: 10.1001/archpsyc.1991.01810280099018. [DOI] [PubMed] [Google Scholar]
- 97.Nielsen DA, Virkkunen M, Lappalainen J, Eggert M, Brown GL, Long JC, et al. A tryptophan hydroxylase gene marker for suicidality and alcoholism. Archives of general psychiatry. 1998;55(7):593–602. doi: 10.1001/archpsyc.55.7.593. [DOI] [PubMed] [Google Scholar]
- 98.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 99.Lesch KP, Gutknecht L. Pharmacogenetics of the serotonin transporter. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(6):1062–1073. doi: 10.1016/j.pnpbp.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 100.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 101.Surtees PG, Wainwright NWJ, Willis-Owen SAG, Luben R, Day NE, Flint J. Social Adversity, the Serotonin Transporter (5-HTTLPR) Polymorphism and Major Depressive Disorder. Biological Psychiatry. 2006;59(3):224–229. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 102.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 103.Jacob CP, Strobel A, Hohenberger K, Ringel T, Gutknecht L, Reif A, et al. Association between allelic variation of serotonin transporter function and neuroticism in anxious cluster C personality disorders. The American journal of psychiatry. 2004;161(3):569–72. doi: 10.1176/appi.ajp.161.3.569. [DOI] [PubMed] [Google Scholar]
- 104.Willis-Owen SA, Turri MG, Munafo MR, Surtees PG, Wainwright NW, Brixey RD, et al. The serotonin transporter length polymorphism, neuroticism, and depression: a comprehensive assessment of association. Biological psychiatry. 2005;58(6):451–6. doi: 10.1016/j.biopsych.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 105.Middeldorp CM, de Geus EJ, Beem AL, Lakenberg N, Hottenga JJ, Slagboom PE, et al. Family Based Association Analyses between the Serotonin Transporter Gene Polymorphism (5-HTTLPR) and Neuroticism, Anxiety and Depression. Behavior genetics. 2007;37(2):294–301. doi: 10.1007/s10519-006-9139-7. [DOI] [PubMed] [Google Scholar]
- 106.Perez de Castro I, Ibanez A, Saiz-Ruiz J, Fernandez-Piqueras J. Genetic contribution to pathological gambling: possible association between a functional DNA polymorphism at the serotonin transporter gene (5-HTT) and affected men. Pharmacogenetics. 1999 Jun;9(3):397–400. [PubMed] [Google Scholar]
- 107.Perez de Castro I, Ibanez A, Saiz-Ruiz J, Fernandez-Piqueras J. Concurrent positive association between pathological gambling and functional DNA polymorphisms at the MAO-A and the 5-HT transporter genes. Mol Psychiatry. 2002;7(9):927–8. doi: 10.1038/sj.mp.4001148. [DOI] [PubMed] [Google Scholar]
- 108.Devor EJ, Magee HJ, Dill-Devor RM, Gabel J, Black DW. Serotonin transporter gene (5-HTT) polymorphisms and compulsive buying. American journal of medical genetics. 1999;88(2):123–5. doi: 10.1002/(sici)1096-8628(19990416)88:2<123::aid-ajmg5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 109.Hemmings SM, Kinnear CJ, Lochner C, Seedat S, Corfield VA, Moolman-Smook JC, et al. Genetic correlates in trichotillomania--A case-control association study in the South African Caucasian population. The Israel journal of psychiatry and related sciences. 2006;43(2):93–101. [PubMed] [Google Scholar]
- 110.Brewer JA, Grant JE, Potenza MN. The Treatment of Pathological Gambling. Addictive Disorders and Their Treatment. in press. [Google Scholar]
- 111.Grant JE, Odlaug BL, Potenza MN. Addicted to Hairpulling? How An Alternative Model of Trichotillomania May Improve Treatment Outcome. Harv Rev Psychiatry. doi: 10.1080/10673220701298407. In Press. [DOI] [PubMed] [Google Scholar]
- 112.Mick TM, Hollander E. Impulsive-compulsive sexual behavior. CNS spectrums. 2006;11(12):944–55. doi: 10.1017/s1092852900015133. [DOI] [PubMed] [Google Scholar]
- 113.Liu T, Potenza MN. Problematic Internet Use - Clinical Implications. CNS Spectr. doi: 10.1017/s1092852900015339. In Press. [DOI] [PubMed] [Google Scholar]
- 114.Hollander E, DeCaria CM, Finkell JN, Begaz T, Wong CM, Cartwright C. A randomized double-blind fluvoxamine/placebo crossover trial in pathologic gambling. Biol Psychiatry. 2000;47(9):813–7. doi: 10.1016/s0006-3223(00)00241-9. [DOI] [PubMed] [Google Scholar]
- 115.Kim SW, Grant JE, Adson DE, Shin YC, Zaninelli R. A double-blind placebo-controlled study of the efficacy and safety of paroxetine in the treatment of pathological gambling. J Clin Psychiatry. 2002;63(6):501–7. doi: 10.4088/jcp.v63n0606. [DOI] [PubMed] [Google Scholar]
- 116.Grant JE, Kim SW, Potenza MN, Blanco C, Ibanez A, Stevens L, et al. Paroxetine treatment of pathological gambling: a multi-centre randomized controlled trial. Int Clin Psychopharmacol. 2003;18(4):243–9. doi: 10.1097/00004850-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 117.Blanco C, Petkova E, Ibanez A, Saiz-Ruiz J. A pilot placebo-controlled study of fluvoxamine for pathological gambling. Ann Clin Psychiatry. 2002;14(1):9–15. doi: 10.1023/a:1015215809770. [DOI] [PubMed] [Google Scholar]
- 118.Wainberg ML, Muench F, Morgenstern J, Hollander E, Irwin TW, Parsons JT, et al. A double-blind study of citalopram versus placebo in the treatment of compulsive sexual behaviors in gay and bisexual men. The Journal of clinical psychiatry. 2006;67(12):1968–73. doi: 10.4088/jcp.v67n1218. [DOI] [PubMed] [Google Scholar]
- 119.Black DW, Gabel J, Hansen J, Schlosser S. A double-blind comparison of fluvoxamine versus placebo in the treatment of compulsive buying disorder. Annals of clinical psychiatry. 2000;12(4):205–11. doi: 10.1023/a:1009030425631. [DOI] [PubMed] [Google Scholar]
- 120.Ninan PT, McElroy SL, Kane CP, Knight BT, Casuto LS, Rose SE, et al. Placebo-controlled study of fluvoxamine in the treatment of patients with compulsive buying. Journal of clinical psychopharmacology. 2000;20(3):362–6. doi: 10.1097/00004714-200006000-00012. [DOI] [PubMed] [Google Scholar]
- 121.Bullock K, Koran L. Psychopharmacology of compulsive buying. Drugs of today (Barcelona, Spain. 2003;39(9):695–700. doi: 10.1358/dot.2003.39.9.799477. [DOI] [PubMed] [Google Scholar]
- 122.Grant JE, Potenza MN. Escitalopram Treatment of Pathological Gambling with Co-Occurring Anxiety: An Open-Label Pilot Study with Double-Blind Discontinuation. Int Clin Psychopharmacol. 2006;21:203–9. doi: 10.1097/00004850-200607000-00002. [DOI] [PubMed] [Google Scholar]
- 123.Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Neurocognitive functions in pathological gambling: a comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction (Abingdon, England) 2006;101(4):534–47. doi: 10.1111/j.1360-0443.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- 124.Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441(7095):876–9. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature neuroscience. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 126.Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54(1):51–8. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 127.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 128.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science (New York, NY. 2003;301(5636):1104–7. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 129.Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nature neuroscience. 2004;7(8):887–93. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- 130.Bechara A. Risky business: emotion, decision-making, and addiction. J Gambl Stud. 2003;19(1):23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- 131.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(13):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 132.Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40(10):1675–89. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 133.Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180–7. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- 134.London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex. 2000;10(3):334–42. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- 135.Adinoff B, Devous MD, Sr, Cooper DB, Best SE, Chandler P, Harris T, et al. Resting regional cerebral blood flow and gambling task performance in cocaine-dependent subjects and healthy comparison subjects. Am J Psychiatry. 2003;160(10):1892–4. doi: 10.1176/appi.ajp.160.10.1892. [DOI] [PubMed] [Google Scholar]
- 136.Tucker KA, Potenza MN, Beauvais JE, Browndyke JN, Gottschalk PC, Kosten TR. Perfusion abnormalities and decision making in cocaine dependence. Biol Psychiatry. 2004;56(7):527–30. doi: 10.1016/j.biopsych.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 137.Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, Banich MT. Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision-making. 2007 doi: 10.1002/hbm.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Petry NM. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. J Abnorm Psychol. 2001;110(3):482–7. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- 139.Potenza MN, Leung HC, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, et al. An FMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiatry. 2003;160(11):1990–4. doi: 10.1176/appi.ajp.160.11.1990. [DOI] [PubMed] [Google Scholar]
- 140.Reuter J, Raedler T, Rose M, Hand I, Glascher J, Buchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nature Neuroscience. 2005;8(2):147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- 141.Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20(4):322–39. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 142.Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, et al. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144(4):1153–9. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Excitatory transmission in the basolateral amygdala. Journal of neurophysiology. 1991;66(3):986–98. doi: 10.1152/jn.1991.66.3.986. [DOI] [PubMed] [Google Scholar]
- 144.Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Inhibitory transmission in the basolateral amygdala. Journal of neurophysiology. 1991;66(3):999–1009. doi: 10.1152/jn.1991.66.3.999. [DOI] [PubMed] [Google Scholar]
- 145.Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. The Journal of neuroscience. 2004;24(20):4718–22. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19(13):5473–81. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bechara A. Disturbances of emotion regulation after focal brain lesions. International review of neurobiology. 2004;62:159–93. doi: 10.1016/S0074-7742(04)62006-X. [DOI] [PubMed] [Google Scholar]
- 148.Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Annals of the New York Academy of Sciences. 1999;877:412–38. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 149.Bechara A. Neurobiology of decision-making: risk and reward. Seminars in clinical neuropsychiatry. 2001;6(3):205–16. doi: 10.1053/scnp.2001.22927. [DOI] [PubMed] [Google Scholar]
- 150.Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. I. Frontal cortex and attention to action. Journal of neurophysiology. 1997;77(3):1313–24. doi: 10.1152/jn.1997.77.3.1313. [DOI] [PubMed] [Google Scholar]
- 151.Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. II. Subcortical structures and learning by trial and error. Journal of neurophysiology. 1997;77(3):1325–37. doi: 10.1152/jn.1997.77.3.1325. [DOI] [PubMed] [Google Scholar]
- 152.Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. The Journal of neuroscience. 2004;24(14):3554–62. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Holland PC. Relations between Pavlovian-instrumental transfer and reinforcer devaluation. Journal of experimental psychology. 2004;30(2):104–17. doi: 10.1037/0097-7403.30.2.104. [DOI] [PubMed] [Google Scholar]
- 154.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. The Journal of neuroscience. 2000;20(6):2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. The Journal of neuroscience. 2005;25(38):8665–70. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. The American journal of psychiatry. 2002;159(10):1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, et al. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27(1):35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- 158.Stein DJ, Chamberlain SR, Fineberg N. An A-B-C model of habit disorders: hair-pulling, skin-picking, and other stereotypic conditions. CNS spectrums. 2006;11(11):824–7. doi: 10.1017/s1092852900014978. [DOI] [PubMed] [Google Scholar]
- 159.Potenza MN, Gottschalk C, Skudlarski P, Fulbright RK, Lacadie CM, Wilber MK, et al. College on Problems of Drug Dependence. Orlando, FL: 2005. fMRI of Craving States in Pathological Gambling and Cocaine Dependence. [Google Scholar]
- 160.O’Sullivan RL, Rauch SL, Breiter HC, Grachev ID, Baer L, Kennedy DN, et al. Reduced basal ganglia volumes in trichotillomania measured via morphometric magnetic resonance imaging. Biological Psychiatry. 1997;42(1):39–45. doi: 10.1016/S0006-3223(96)00297-1. [DOI] [PubMed] [Google Scholar]
- 161.Wallace BC. Psychological and environmental determinants of relapse in crack cocaine smokers. J Subst Abuse Treat. 1989;6(2):95–106. doi: 10.1016/0740-5472(89)90036-6. [DOI] [PubMed] [Google Scholar]
- 162.Bradley BP, Phillips G, Green L, Gossop M. Circumstances surrounding the initial lapse to opiate use following detoxification. Br J Psychiatry. 1989;154:354–9. doi: 10.1192/bjp.154.3.354. [DOI] [PubMed] [Google Scholar]
- 163.Cabib S, Puglisi-Allegra S, Genua C, Simon H, Le Moal M, Piazza PV. Dose-dependent aversive and rewarding effects of amphetamine as revealed by a new place conditioning apparatus. Psychopharmacology (Berl) 1996;125(1):92–6. doi: 10.1007/BF02247398. [DOI] [PubMed] [Google Scholar]
- 164.Kalivas PW, Duffy P. Similar effects of daily cocaine and stress on mesocorticolimbic dopamine neurotransmission in the rat. Biol Psychiatry. 1989;25(7):913–28. doi: 10.1016/0006-3223(89)90271-0. [DOI] [PubMed] [Google Scholar]
- 165.Ramsey NF, Van Ree JM. Emotional but not physical stress enhances intravenous cocaine self-administration in drug-naive rats. Brain Res. 1993;608(2):216–22. doi: 10.1016/0006-8993(93)91461-z. [DOI] [PubMed] [Google Scholar]
- 166.Nash JF, Jr, Maickel RP. The role of the hypothalamic-pituitary-adrenocortical axis in post-stress induced ethanol consumption by rats. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12(5):653–71. doi: 10.1016/0278-5846(88)90010-3. [DOI] [PubMed] [Google Scholar]
- 167.Volpicelli JR. Uncontrollable events and alcohol drinking. Br J Addict. 1987;82(4):381–92. doi: 10.1111/j.1360-0443.1987.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 168.Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry. 2005;162(8):1483–93. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- 169.Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170(1):62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- 170.Baumann MH, Gendron TM, Becketts KM, Henningfield JE, Gorelick DA, Rothman RB. Effects of intravenous cocaine on plasma cortisol and prolactin in human cocaine abusers. Biological psychiatry. 1995;38(11):751–5. doi: 10.1016/0006-3223(95)00083-6. [DOI] [PubMed] [Google Scholar]
- 171.Rivier C, Vale W. Cocaine stimulates adrenocorticotropin (ACTH) secretion through a corticotropin-releasing factor (CRF)-mediated mechanism. Brain research. 1987;422(2):403–6. doi: 10.1016/0006-8993(87)90953-x. [DOI] [PubMed] [Google Scholar]
- 172.Swerdlow NR, Koob GF, Cador M, Lorang M, Hauger RL. Pituitary-adrenal axis responses to acute amphetamine in the rat. Pharmacology, biochemistry, and behavior. 1993;45(3):629–37. doi: 10.1016/0091-3057(93)90518-x. [DOI] [PubMed] [Google Scholar]
- 173.Mendelson JH, Ogata M, Mello NK. Adrenal function and alcoholism. I. Serum cortisol. Psychosomatic medicine. 1971;33(2):145–57. doi: 10.1097/00006842-197103000-00006. [DOI] [PubMed] [Google Scholar]
- 174.Sarnyai Z, Shaham Y, Heinrichs SC. The Role of Corticotropin-Releasing Factor in Drug Addiction. Pharmacol Rev. 2001;53(2):209–244. [PubMed] [Google Scholar]
- 175.McIntyre IM, Norman TR, Burrows GD, Armstrong SM. Alterations to plasma melatonin and cortisol after evening alprazolam administration in humans. Chronobiology international. 1993;10(3):205–13. doi: 10.3109/07420529309073889. [DOI] [PubMed] [Google Scholar]
- 176.Imperato A, Angelucci L, Casolini P, Zocchi A, Puglisi-Allegra S. Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain research. 1992;577(2):194–9. doi: 10.1016/0006-8993(92)90274-d. [DOI] [PubMed] [Google Scholar]
- 177.McCullough LD, Salamone JD. Involvement of nucleus accumbens dopamine in the motor activity induced by periodic food presentation: a microdialysis and behavioral study. Brain research. 1992;592(12):29–36. doi: 10.1016/0006-8993(92)91654-w. [DOI] [PubMed] [Google Scholar]
- 178.Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, et al. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183(2):171–80. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- 179.Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: does self-control resemble a muscle? Psychological bulletin. 2000;126(2):247–59. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- 180.Brewer JA, Grant JE, Potenza MN. The Neurobiology of Pathological Gambling. In: Smith G, Hodgins D, Williams R, editors. Research and Measurement Issues in Gambling Studies. Elsivier; San Diego: In Press. [Google Scholar]
- 181.Meyer G, Hauffa BP, Schedlowski M, Pawlak C, Stadler MA, Exton MS. Casino gambling increases heart rate and salivary cortisol in regular gamblers. Biological Psychiatry. 2000;48(9):948–953. doi: 10.1016/s0006-3223(00)00888-x. [DOI] [PubMed] [Google Scholar]
- 182.Krueger THC, Schedlowski M, Meyer G. Cortisol and Heart Rate Measures during Casino Gambling in Relation to Impulsivity. Neuropsychobiology. 2005;52(4):206–211. doi: 10.1159/000089004. [DOI] [PubMed] [Google Scholar]
- 183.Meyer G, Schwertfeger J, Exton MS, Janssen OE, Knapp W, Stadler MA, et al. Neuroendocrine response to casino gambling in problem gamblers. Psychoneuroendocrinology. 2004;29(10):1272–1280. doi: 10.1016/j.psyneuen.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 184.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12(2):483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. The Journal of neuroscience. 2003;23(31):9981–6. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. The Journal of neuroscience. 2006;26(10):2788–97. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hall FS, Li XF, Goeb M, Roff S, Hoggatt H, Sora I, et al. Congenic C57BL/6 mu opiate receptor (MOR) knockout mice: baseline and opiate effects. Genes, brain, and behavior. 2003;2(2):114–21. doi: 10.1034/j.1601-183x.2003.00016.x. [DOI] [PubMed] [Google Scholar]
- 188.Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(16):9608–13. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Oslin DW, Berrettini WH, O’Brien CP. Targeting treatments for alcohol dependence: the pharmacogenetics of naltrexone. Addiction biology. 2006;11(34):397–403. doi: 10.1111/j.1369-1600.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 190.Shinohara K, Yanagisawa A, Kagota Y, Gomi A, Nemoto K, Moriya E, et al. Physiological changes in Pachinko players; beta-endorphin, catecholamines, immune system substances and heart rate. Appl Human Sci. 1999;18(2):37–42. doi: 10.2114/jpa.18.37. [DOI] [PubMed] [Google Scholar]
- 191.Tamminga CA, Nestler EJ. Pathological gambling: focusing on the addiction, not the activity. Am J Psychiatry. 2006;163(2):180–1. doi: 10.1176/appi.ajp.163.2.180. [DOI] [PubMed] [Google Scholar]
- 192.O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162(8):1423–31. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 193.Kim SW, Grant JE, Adson DE, Shin YC. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914–21. doi: 10.1016/s0006-3223(01)01079-4. [DOI] [PubMed] [Google Scholar]
- 194.Grant JE, Potenza MN, Hollander E, Cunningham-Williams R, Nurminen T, Smits G, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303–12. doi: 10.1176/appi.ajp.163.2.303. [DOI] [PubMed] [Google Scholar]
- 195.Raymond NC, Grant JE, Kim SW, Coleman E. Treatment of compulsive sexual behaviour with naltrexone and serotonin reuptake inhibitors: two case studies. International clinical psychopharmacology. 2002;17(4):201–5. doi: 10.1097/00004850-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 196.Ryback RS. Naltrexone in the treatment of adolescent sexual offenders. The Journal of clinical psychiatry. 2004;65(7):982–6. doi: 10.4088/jcp.v65n0715. [DOI] [PubMed] [Google Scholar]
- 197.Braff DL, Freedman R, Schork NJ, Gottesman II. Deconstructing Schizophrenia: An Overview of the Use of Endophenotypes in Order to Understand a Complex Disorder. Schizophr Bull. 2007;33(1):21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gottesman II, Gould TD. The Endophenotype Concept in Psychiatry: Etymology and Strategic Intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]


