SecDF is a multi-path membrane protein required for efficient protein translocation and integration via translocon. Purification and crystallization of T. thermophilus SecDF have been achieved by exploiting unique crystallization techniques that allowed the collection of a 3.74 Å data set.
Keywords: SecDF, membrane proteins, protein translocation, dehydration
Abstract
Thermus thermophilus has a multi-path membrane protein, TSecDF, as a single-chain homologue of Escherichia coli SecD and SecF, which form a translocon-associated complex required for efficient preprotein translocation and membrane-protein integration. Here, the cloning, expression in E. coli, purification and crystallization of TSecDF are reported. Overproduced TSecDF was solubilized with dodecylmaltoside, chromatographically purified and crystallized by vapour diffusion in the presence of polyethylene glycol. The crystals yielded a maximum resolution of 4.2 Å upon X-ray irradiation, revealing that they belonged to space group P43212. Attempts were made to improve the diffraction quality of the crystals by combinations of micro-stirring, laser-light irradiation and dehydration, which led to the eventual collection of complete data sets at 3.74 Å resolution and preliminary success in the single-wavelength anomalous dispersion analysis. These results provide information that is essential for the determination of the three-dimensional structure of this important membrane component of the protein-translocation machinery.
1. Introduction
The Sec protein-translocation machinery provides a channel-like pathway for the movement of newly synthesized secretory and membrane proteins across and into the plasma membrane (in prokaryotic cells) or the endoplasmic reticulum membrane (in eukaryotic cells). The core component of the protein-conducting channel, called the translocon, is composed of an evolutionarily conserved heterotrimeric membrane-protein complex, SecYEG in prokaryotes and Sec61αβγ in eukaryotes (van den Berg et al., 2004 ▶; Osborne et al., 2005 ▶; Veenendaal et al., 2004 ▶). An X-ray crystallographic structure has been determined for an archaeal SecYEβ complex (van den Berg et al., 2004 ▶). In this structure, the N-terminal and the C-terminal halves of SecY are arranged pseudosymmetrically with an hour-glass-shaped narrow channel in the centre, which is gated vertically by a ‘plug helix’ and laterally by a presumed flexible ‘front’ interface between the SecY halves, which are hinged and backed up by SecE on the ‘back’ side.
Although evidence indicates that the translocating polypeptide is in close contact with the channel region of the Escherichia coli SecYEG protomer (Cannon et al., 2005 ▶), much more information needs to be elucidated before we can fully understand the working structure of the translocon. For instance, further oligomerization of the heterotrimer is possible and a front-to-front dimer assembly has been reported to be induced by attachment of SecYEG to the translating ribosome (Mitra et al., 2005 ▶). E. coli SecYEG associates further with the cytosolic motor protein SecA (Vrontou & Economou, 2004 ▶) and with the SecDF–YajC complex within the membrane (Duong & Wickner, 1997 ▶).
The importance of SecDF in protein translocation was shown by the severe growth and protein-export defects associated with genetic depletion of these proteins (Pogliano & Beckwith, 1994a ▶). Conversely, its overproduction compensated for cellular protein-export defects, including those caused by signal-sequence deficiency (Pogliano & Beckwith, 1994a ▶). SecDF is required in vitro for a high capacity of translocation activity by SecYEG/SecA (Nouwen et al., 2005 ▶). In the past, several different action points have been proposed for SecDF including (i) facilitation of the substrate release to the periplasmic side (Matsuyama et al., 1993 ▶), (ii) maintenance of the membrane potential (Arkowitz & Wickner, 1994 ▶), (iii) stabilization of the ‘membrane-inserted’ state of SecA (Kim et al., 1994 ▶; Economou et al., 1995 ▶) and (iv) facilitation of the membrane-protein integration process by bridging SecYEG and YidC (Nouwen & Driessen, 2002 ▶; Chen et al., 2005 ▶). Cleary, its full understanding requires structural information. Currently, we only know that each of the E. coli SecD and SecF proteins contains six transmembrane segments and a characteristically large first periplasmic domain (Pogliano & Beckwith, 1994b ▶), which is indeed important functionally (Nouwen et al., 2005 ▶).
Prompted by the possible advantages provided by thermal stability of proteins from thermophilic bacteria, we set up an experimental system to characterize the Thermus thermophilus protein-translocation components TSecY–TSecE and TSecA (Mori, Tsukazaki et al., 2003 ▶). It was noted that, as in Bacillus subtilis (Bolhuis et al., 1998 ▶), the T. thermophilus genome contains a single open reading frame containing both SecD- and SecF-equivalent sequences (R. Masui & S. Kuramitus, unpublished work; Henne et al., 2004 ▶). This single-chain protein (TSecDF) is predicted to consist of 735 amino-acid residues (molecular weight of 79.7 kDa) and to contain 12 transmembrane segments. Here, we report the cloning, purification, crystallization and preliminary X-ray diffraction analyses of TSecDF.
2. Material, methods and results
2.1. Cloning of secDF from T. thermophilus
The secDF gene (gene ID 3168575) was amplified from T. thermophilus HB8 genomic DNA (purchased from Takara Shuzo), using Pfx DNA polymerase (Invitrogen) and primers 5′-CTGCCATGGACCGGAAAAACCTCACCAG-3′ and 5′-GACGGTACCCTAATGGTGATGGTGATGGTGGGCCTTGCTGGCCTCTTGG-3′. The upstream primer contained the NcoI-recognition sequence (in italics in the first sequence) to enable in-frame fusion of the TSecDF-coding region to the second codon of lacZα on the cloning vector which also contained the NcoI site. However, because the G residue (indicated in bold-italic) deviated from the secDF sequence, an Asn to Asp change was introduced into the second residue of the cloned TSecDF. The downstream primer contained the KpnI-recognition sequence (bold in the second sequence) as well as a segment (italicized) directing a His6 sequence attached to the C-terminus. The amplified DNA was digested with NcoI and KpnI and cloned into a lac promoter vector, pTV118N (Takara Shuzo), which was also treated with the same restriction enzymes. A resulting recombinant plasmid, with confirmed nucleotide sequences for the lac promoter-secDF-his 6 arrangement, was named pTT206. The protein we purified for crystallization had the amino-acid sequence Met1-Asp2-Arg3…Lys735-His 6, in which residues deviating from the native primary sequence of TSecDF (Met1-Asn2-Arg3…Lys735) are italicized.
2.2. Purification of TSecDF
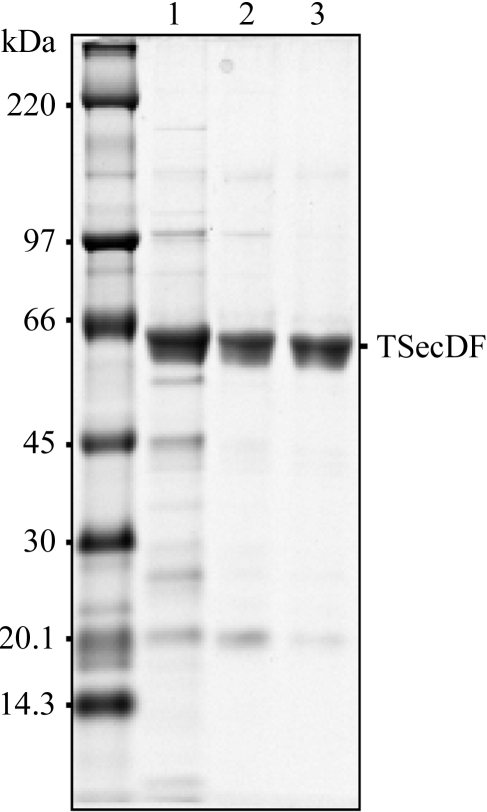
Plasmid pTT206 (TSecDF) was introduced into E. coli strain AD202 (Akiyama & Ito, 1990 ▶) which additionally harboured pSTD343 (lacI q; Mori, Akiyama et al., 2003 ▶). Cells were then precultured at 310 K in 100 ml LB medium supplemented with 50 µg ml−1 ampicillin, 20 µg ml−1 chloramphenicol and 0.4%(w/v) glucose and were inoculated into 10 l LB medium containing the same antibiotics and 1 mM isopropyl 1-thio-β-d-galactopyranoside for additional growth at 310 K for 12 h. Cells were harvested by centrifugation and total membranes were prepared as described previously (Matsuo et al., 1998 ▶) and solubilized with 20 mM Tris–HCl buffer pH 8.1 containing 2%(w/v) n-dodecyl-β-maltoside (DDM), 5%(v/v) glycerol, 300 mM NaCl, 20 mM imidazole pH 7.0 and 0.1 mM Pefabloc (Merck) by gentle mixing for 30 min at 277 K. After the removal of insoluble materials by ultracentrifugation, the supernatant was applied onto an Ni–NTA column equilibrated with buffer A [20 mM Tris–HCl pH 8.1, 0.1%(w/v) DDM, 5%(v/v) glycerol, 300 mM NaCl and 0.1 mM Pefabloc]. The sample was then eluted with a 20–300 mM imidazole gradient in buffer A. Fractions containing TSecDF (Fig. 1 ▶, lane 1; confirmed for its identity by anti-His6 immunoblotting as well as by mass-spectroscopic analysis) were pooled and concentrated by ultrafiltration with Amicon Ultra 30K (Millipore), followed by desalting by passing through a Hi-Trap desalting column (Amersham Biosciences) that was pre-equilibrated with buffer B [20 mM Tris–HCl pH 8.1, 0.1%(w/v) DDM, 5%(v/v) glycerol and 0.1 mM Pefabloc]. The sample was then separated by Hi-Trap SP (Amersham Biosciences) cation-exchange column chromatography, eluting with a 0–1 M linear NaCl gradient in buffer B. At this stage, the preparation was at least 90% pure as estimated by SDS–PAGE and Coomassie brilliant blue staining (Fig. 1 ▶, lane 2). For further purification, the SecDF fractions were concentrated and subjected to Superose 6 (Amersham Biosciences) size-exclusion column chromatography, eluting with buffer A. Typically, we obtained ∼1.5 mg purified TSecDF from a 10 l culture (Fig. 1 ▶, lane 3). Protein concentration was estimated by assuming an A 280 of 0.68 for a 1 mg ml−1 solution.
Figure 1.
Purification of TSecDF. During the TSecDF purification procedures, samples were examined by 10% SDS–PAGE using the tricine buffer system (Schagger & von Jagow, 1987 ▶) and Coomassie brilliant blue staining. Lane 1, Ni–NTA affinity chromatography step; lane 2, cation-exchange chromatography step; lane 3, gel-filtation step.
For preparation of SeMet-labelled TSecDF, the TSecDF-overproducing cells (AD202/pTT206/pSTD343) were pre-cultured as described above, washed three times with 0.9% NaCl and then inoculated into 10 l minimal-salt medium [5.6 g K2HPO4, 1.6 g KH2PO4, 0.8 g (NH4)2SO4, 0.2 g sodium citrate, 4.2 mg FeSO4, 80 mg MgSO4.7H2O and 20 ml glycerol per litre], supplemented with the same antibiotics as above, 1 mM isopropyl 1-thio-β-d-galactopyranoside, 1× Kao and Michayluk vitamins (Sigma) and the following amino acids: 40 µg ml−1 each of Ala, Arg, Asn, Asp, Cys, Gln, Glu, Gly, His, Pro, Ser, Trp and Tyr, 100 µg ml−1 each of Ile, Leu, Lys, Phe, Thr and Val and 25 µg ml−1 SeMet. The SeMet form of TSecDF was purified by the same procedures as used for purification of the native TSecDF. SeMet should have been incorporated into the seven Met positions of the recombinant TSecDF (see above for the exact amino-acid sequence). The yield was approximately 0.25 mg.
2.3. Crystallization of TSecDF
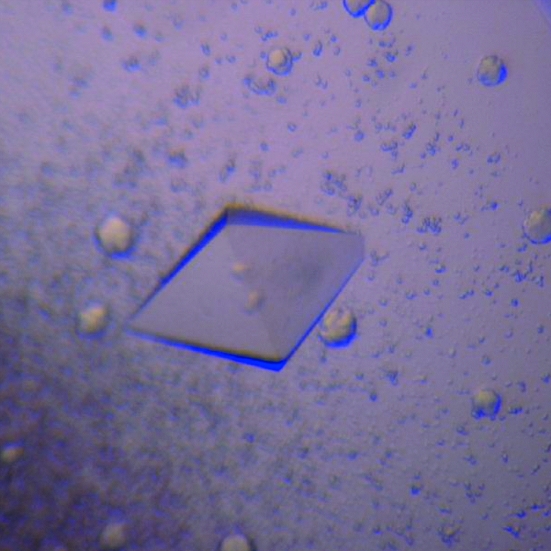
A purified sample of TSecDF was concentrated to approximately 10 mg ml−1 and dialyzed against 0.02% DDM. Initially, crystallization conditions were searched for using sitting-drop vapour diffusion in combination with the JB Screen kit (Jena Bioscience). 1 µl drops of TSecDF solution (8 mg ml−1) were mixed with 0.5 µl of the respective reservoir solutions and equilibrated at 293 K against 70–500 µl reservoir solution. Small crystals appeared after a few days with reservoir solutions consisting of 30% PEG 400, 0.1 M MES pH 6.5 and 0.1 M sodium acetate or of 20% PEG 1500 and 0.1 M HEPES pH 7.5. After further optimization of reservoir conditions, octahedral crystals appeared and grew to dimensions of 0.3 × 0.18 × 0.18 mm in 2–3 weeks with reservoir solution containing 26% PEG 400 and 0.2 M sodium acetate (Fig. 2 ▶). The crystals were stable, maintaining a good-looking shape in the drop at least for three months. The crystal with the best diffraction quality (native III; Table 1 ▶) was obtained using 70 µl reservoir solution with 26% PEG 400 and 0.2 M sodium acetate followed by the additional manipulations described below.
Figure 2.
Crystal of TSecDF. A TSecDF crystal obtained by hanging-drop vapour diffusion with reservoir solution containing 26% PEG 400 and 0.2 M sodium acetate is shown. The crystal dimensions are approximately 0.3 × 0.18 × 0.18 mm.
Table 1. Summary of data collection for TSecDF crystals.
Values in parentheses are for the last shell.
| Native I | Native II | Native III | SeMet | |
|---|---|---|---|---|
| Special conditions | Micro-stirring/laser irradiation | Dehydration | Micro-stirring/laser irradition | |
| Crystal dimensions (mm) | 0.20 × 0.12 × 0.12 | 0.5 × 0.3 × 0.3 | 0.4 × 0.24 × 0.24 | 0.35 × 0.2 × 0.2 |
| Wavelength (Å) | 1.00000 | 1.00000 | 1.00000 | 0.97874 |
| Crystal-to-detector distance (mm) | 430 | 450 | 450 | 500 |
| Total oscillation angle (°) | 180 | 350 | 350 | 300 |
| Oscillation range (°) | 1 | 1 | 1 | 1 |
| Exposure time (s) | 4 | 10 | 10 | 10 |
| Detector | ADSC Q-315 | ADSC Q-315 | ADSC Q-315 | ADSC Q-315 |
| Space group | P43212 | P43212 | P43212 | P43212 |
| Unit-cell parameters (Å) | ||||
| a | 140.7 | 141.6 | 138.8 | 140.4 |
| b | 140.7 | 141.6 | 138.8 | 140.4 |
| c | 160.8 | 161.1 | 160.6 | 159.9 |
| Resolution (Å) | 50–4.20 (4.35–4.20) | 50–4.20 (4.27–4.20) | 50–3.74 (3.80–3.74) | 50–4.40 (4.48–4.40) |
| Observed reflections | 120485 | 229029 | 291468 | 158492 |
| Unique reflections | 12228 | 12401 | 16751 | 10492 |
| Redundancy | 9.85 | 18.5 | 17.4 | 15.2 |
| Completeness (%) | 99.2 (99.1) | 99.2 (99.2) | 99.6 (100) | 98.7 (98.8) |
| I/σ(I) | 32.7 (3.20) | 54.8 (3.76) | 40.2 (2.24) | 49.4 (3.98) |
| Rsym† | 0.050 (0.341) | 0.061 (0.443) | 0.075 (0.571) | 0.074 (0.512) |
R
sym = 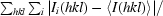
 .
.
To improve the diffraction quality and to increase the size of TSecDF crystals, we used the micro-stirring technique and the laser-irradiated growth technique (Adachi, Takano, Hosokawa et al., 2003 ▶; Adachi, Takano, Yoshimura et al., 2003 ▶; Adachi et al., 2005 ▶) provided by SOSHO Inc. A specially designed crystallization plate was used to irradiate a laser beam through the bottom of the plate and to grow crystals using the sitting-drop vapour-diffusion technique. The femtosecond laser beam (IFRIT Cyber Laser) was focused on the TSecDF drop using an objective lens. The drop was then incubated with shaking. As an end result, we obtained larger TSecDF crystals with well suppressed mosaicity, as described below.
2.4. Preliminary data collection and processing
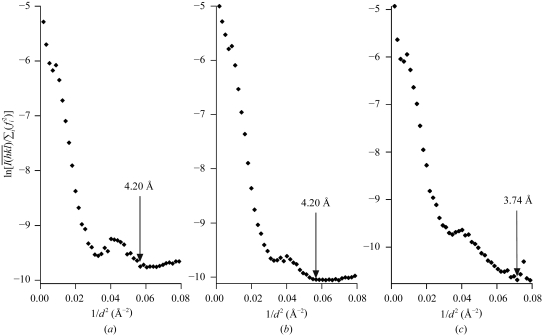
The initial series of TSecDF crystals were transferred into a cryoprotectant solution containing 35% PEG 400, 0.2 M sodium acetate and 0.02% DDM. The treated crystals were then flash-cooled in a nitrogen-gas stream at 100 K and irradiated with a synchrotron beam (BL41XU, SPring-8, Harima, Japan). The X-ray diffraction data were processed using the DENZO and SCALEPACK (Otwinowski & Minor, 1997 ▶) programs and the effective resolution of the diffraction was estimated from Wilson plots (Fig. 3 ▶, arrows). Thus, X-ray diffraction patterns to 4.2 Å resolution were initially obtained (Table 1 ▶, native I; Fig. 3 ▶ a). However, almost all the crystals obtained after the sitting-drop procedure displayed serious mosaicity of 1.5° or higher. In contrast, more than 50% of crystals generated by the hanging-drop technique had mosaicity of less than 1.0°. Furthermore, the micro-stirring and the laser-irradiation techniques (Adachi, Takano, Hosokawa et al., 2003 ▶; Adachi, Takano, Yoshimura et al., 2003 ▶; Adachi et al., 2005 ▶; SOSHO Inc.) not only reduced the crystal mosaicity, but also increased the crystal size. Thus, we obtained crystals with a maximum dimension of 0.5 mm, which were transferred to the cryoprotectant solution and flash-cooled. They diffracted X-rays to 4.2 Å resolution with higher diffraction intensity (Table 1 ▶, native II; Fig. 3 ▶ b). The crystals proved to be tetragonal, with space group P43212 and unit-cell parameters a = b = 141.6, c = 161.1 Å. The unit cell contained one TSecDF molecule (MW 80.5 kDa), giving a Matthews coefficient V M of 5.02 Å3 Da−1 and a solvent content of 75.5%. It seems unlikely that the unit cell contained more than one TSecDF molecule, since the apparent molecular weight of solubilized and detergent-bound TSecDF monomer was estimated to be approximately 150 kDa.
Figure 3.
Wilson plots of the X-ray diffraction spots for TSecDF crystals obtained under different crystallization conditions. (a) A crystal obtained under the initial conditions described in the text; (b) a crystal obtained after micro-stirring and laser-light irradiation; (c) a crystal obtained as in (b) but after an additional step of dehydration.
Because of the high solvent content, we subsequently attempted to dehydrate the crystals. After the maximum growth of a crystal, the PEG 400 concentration of the reservoir solution was increased from 26 to 50% in a stepwise manner over 10 h. The dehydrated crystals were soaked in a cryoprotectant solution containing 50% PEG 400, 0.2 M sodium acetate and 0.01% DDM for flash-cooling. Although most crystals cracked during the dehydration procedures, crystals thus manipulated that remained intact had a low mosaicity and diffracted X-rays to 3.74 Å resolution, showing decreased unit-cell parameters and a decreased solvent content (74.4%) (Table 1 ▶, native III; Fig. 3 ▶ c). When we estimated the effective resolutions of natives I, II and III on the basis of I/σ(I) (>2.0), they were 3.77, 3.50 and 3.50 Å, respectively. However, the R sym values at those resolutions diverged seriously. Therefore, it was more appropriate to use Wilson plots for the assessment of the effective resolutions.
In order to solve the three-dimensional structure of TSecDF, we prepared crystals of SeMet-labelled TSecDF using basically the same crystallization conditions (except that the PEG concentration was 27%), including the micro-stirring and the laser-irradiation procedures. The SeMet TSecDF crystals belonged to space group P43212 and diffracted X-rays to 4.4 Å resolution (Table 1 ▶, SeMet). We have already identified the six locations of Se using the program SnB (Miller et al., 1994 ▶, Weeks & Miller, 1999 ▶) and obtained a preliminary electron-density map of TSecDF by means of single-wavelength anomalous diffraction analysis.
Acknowledgments
We thank Tomomi Sakamoto for her able assistance in the purification of TSecDF, Kenji Inaba and Yoshinori Akiyama for helpful suggestions, Kiyoko Mochizuki, Michiyo Sano and Takuhiko Adachi for technical support and Masahide Kawamoto and Nobutaka Shimizu (Japan Synchrotoron Radiation Research Institute) for their help in data collection at SPring-8. This work was supported by CREST (KI and YM) and PREST (ON and SM), Japan Science and Technology Agency, the Industrial Technology Research Grant Program from the New Energy and Industrial Technology Development Organization of Japan (TI) and grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to KI and ON) and its National Project on Protein Structural and Functional Analyses (to KI, ON and SF).
References
- Adachi, H., Niino, A., Murakami, S., Takano, K., Matsumura, H., Kinoshita, T., Warizaya, M., Inoue, T., Mori, Y. & Sasaki, T. (2005). Jpn J. Appl. Phys.44, 1365–1366.
- Adachi, H., Takano, K., Hosokawa, Y., Inoue, T., Mori, Y., Matsumura, H., Yoshimura, M., Tsunaka, Y., Morikawa, M., Kanaya, S., Masuhara, H., Kai, Y. & Sasaki, T. (2003). Jpn J. Appl. Phys.42, L798–L800.
- Adachi, H., Takano, K., Yoshimura, M., Mori, Y. & Sasaki, T. (2003). Jpn J. Appl. Phys.42, L314–L315.
- Akiyama, Y. & Ito, K. (1990). Biochem. Biophys. Res. Commun.167, 711–715. [DOI] [PubMed] [Google Scholar]
- Arkowitz, R. A. & Wickner, W. (1994). EMBO J.13, 954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, B. van den, Clemons, W. M. Jr, Collinson, I., Modis, Y., Hartmann, E., Harrison, S. C. & Rapoport, T. A. (2004). Nature (London), 427, 36–44. [DOI] [PubMed] [Google Scholar]
- Bolhuis, A., Broekhuizen, C. P., Sorokin, A., van Roosmalen, M. L., Venema, G., Bron, S., Quax, W. J. & van Dijl, J. M. (1998). J. Biol. Chem.273, 21217–21224. [DOI] [PubMed] [Google Scholar]
- Cannon, K. S., Or, E., Clemons, W. M. Jr, Shibata, Y. & Rapoport, T. A. (2005). J. Cell Biol.169, 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M., Xie, K., Yuan, J., Yi, L., Facey, S. J., Pradel, N., Wu, L. F., Kuhn, A. & Dalbey, R. E. (2005). Biochemistry, 44, 10741–10749. [DOI] [PubMed] [Google Scholar]
- Duong, F. & Wickner, W. (1997). EMBO J.16, 4871–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou, A., Pogliano, J. A., Beckwith, J., Oliver, D. B. & Wickner, W. (1995). Cell, 83, 1171–1181. [DOI] [PubMed] [Google Scholar]
- Henne, A. et al. (2004). Nature Biotechnol.22, 547–553. [DOI] [PubMed]
- Kim, Y. J., Rajapandi, T. & Oliver, D. (1994). Cell, 78, 845–853. [DOI] [PubMed] [Google Scholar]
- Matsuo, E., Mori, H., Shimoike, T. & Ito, K. (1998). J. Biol. Chem.273, 18835–18840. [DOI] [PubMed] [Google Scholar]
- Matsuyama, S., Fujita, Y. & Mizushima, S. (1993). EMBO J.12, 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, R., Gallo, S. M., Khalak, H. G. & Weeks, C. M. (1994). J. Appl. Cryst.27, 613–621. [Google Scholar]
- Mitra, K., Schaffitzel, C., Shaikh, T., Tama, F., Jenni, S., Brooks, C. L., Ban, N. & Frank, J. (2005). Nature (London), 438, 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, H., Akiyama, Y. & Ito, K. (2003). J. Bacteriol.185, 948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, H., Tsukazaki, T., Masui, R., Kuramitsu, S., Yokoyama, S., Johnson, A. E., Kimura, Y., Akiyama, Y. & Ito, K. (2003). J. Biol. Chem.278, 14257–14264. [DOI] [PubMed] [Google Scholar]
- Nouwen, N. & Driessen, A. J. (2002). Mol. Microbiol.44, 1397–1405. [DOI] [PubMed] [Google Scholar]
- Nouwen, N., Piwowarek, M., Berrelkamp, G. & Driessen, A. J. (2005). J. Bacteriol.187, 5857–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne, A. R., Rapoport, T. A. & van den Berg, B. (2005). Annu. Rev. Cell Dev. Biol.21, 529–550. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Pogliano, J. A. & Beckwith, J. (1994a). EMBO J.13, 554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano, K. J. & Beckwith, J. (1994b). J. Bacteriol.176, 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagger, H. & von Jagow, G. (1987). Anal. Biochem.166, 368–379. [DOI] [PubMed] [Google Scholar]
- Veenendaal, A. K., van der Does, C. & Driessen, A. J. (2004). Biochim. Biophys. Acta, 1694, 81–95. [DOI] [PubMed] [Google Scholar]
- Vrontou, E. & Economou, A. (2004). Biochim. Biophys. Acta, 1694, 67–80. [DOI] [PubMed] [Google Scholar]
- Weeks, C. M. & Miller, R. (1999). Acta Cryst. D55, 492–500. [DOI] [PubMed] [Google Scholar]