Abstract
Corticotropin-releasing hormone (CRH) is a central regulator of the hormonal stress response, causing stimulation of corticotropin and glucocorticoid secretion. CRH is also widely believed to mediate stress-induced behaviors, implying a broader, integrative role for the hormone in the psychological stress response. Mice lacking the CRH gene exhibit normal stress-induced behavior that is specifically blocked by a CRH type 1 receptor antagonist. The other known mammalian ligand for CRH receptors is urocortin. Normal and CRH-deficient mice have an identical distribution of urocortin mRNA, which is confined to the region of the Edinger–Westphal nucleus, and is absent from regions known to mediate stress-related behaviors. Since the Edinger–Westphal nucleus is not known to project to any brain regions believed to play a role in anxiety-like behavior, an entirely different pathway must be postulated for urocortin in the Edinger–Westphal nucleus to mediate these behaviors in CRH-deficient mice. Alternatively, an unidentified CRH-like molecule other than CRH or urocortin, acting through the CRH receptors in brain regions believed to mediate stress-induced behaviors, may mediate the behavioral response to stress, either alone or in concert with CRH.
Stress, defined as the response of the body to any threatening demand (1), can be broadly separated into physiological responses, including the stimulation of adrenal glucocorticoid secretion, and behavioral responses, including anxiety and fearful behavior. Alterations in the stress-response system are believed to underlie many anxiety-related disorders (2–5). Corticotropin-releasing hormone (CRH) has been implicated in both physiological and behavioral stress responses. CRH was identified by its ability to stimulate adrenocorticotropic hormone (ACTH) secretion from anterior pituitary corticotrophs, thus activating the hypothalamic–pituitary–adrenal (HPA) axis (6). In addition, infusion of CRH into the brain was found to cause stress-like behaviors (7), suggesting that CRH integrates physiological and behavioral activities into a generalized stress response. Subsequently, many other pharmacological studies have implicated CRH in the behavioral response to stressors. These studies confirmed that intracerebral infusion of CRH induces stress-like behaviors and additionally that intracerebral infusions of CRH antagonists blunt the behavioral response to a stressor (8–10). Furthermore, transgenic mice that overexpress CRH exhibit increased anxiety-like behavior (11).
The role of the CRH receptor in the behavioral stress response has been further evaluated. The two known CRH receptors, type 1 and type 2 (12), both consist of a 7-transmembrane helix functionally coupled to adenylate cyclase via Gs. For the most part, the anatomical distributions of the two CRH receptors are distinct, with the type 1 receptor expressed in the central nervous system in regions including neo-, olfactory, and hippocampal cortices, subcortical limbic structures in the septal region and amygdala, certain relay nuclei in the brainstem, and the cerebellum (13). One splice variant of the type 2 receptor is expressed in the periphery, whereas another splice variant is expressed in specific subcortical structures, the lateral septal nuclei, and the hypothalamus (14, 15). Two reports of mice lacking the CRH type 1 receptor have confirmed a role for this receptor in anxiety-related behavior (16, 17). CRH type 1 receptor-deficient mice display decreased anxiety-like behavior in the dark–light emergence task and the elevated-plus maze, both behavioral paradigms thought to measure anxiety in rodents. Both studies conclude that CRH mediates the behavioral responses to stressors by means of the CRH type 1 receptor (16, 17). More recently, a CRH type 1 receptor antagonist has been found to block both the acquisition and expression of stress-induced behaviors in rats (18).
Thus, the CRH type 1 receptor clearly mediates behavioral responses to stress, with the evidence to date implicating CRH as its most likely ligand (9, 10, 19). The other known mammalian ligand for CRH receptors is urocortin (20). Urocortin, acting either alone or in concert with CRH, is a potential mediator of in vivo stress responses, because in the studies cited above, infused or transgenically overexpressed CRH could act at receptor sites normally occupied by urocortin, and CRH antagonists could block urocortin actions as well as those of CRH (21). Therefore, we examined the role of CRH in this pathway by characterizing the behavioral responses to stressors of CRH-deficient (knockout, KO) mice (22) and the effects of CRH antagonists on these responses. We also examined the expression of urocortin mRNA in wild-type (WT) and CRH KO mouse brain to assess whether its distribution provides insight into its potential role in mediating stress-induced behaviors.
MATERIALS AND METHODS
Animals and Materials.
Breeding stock for WT and CRH KO mice used in these studies were derived from heterozygote matings (mixed 129SVJ/C57BL6 background). All CRH KO mice were adult male offspring of homozygous KO male × heterozygote female matings, thereby eliminating the need for prenatal glucocorticoid treatment of the mother (22). All animals were housed under a 12-h–12-h light–dark cycle (lights on at 0700) with free access to food and water. At least 7 days prior to each experiment, all animals were singly housed. All experiments were approved by the Animal Care and Use Committees of Children’s Hospital, Louisiana State University, or Tufts University.
CRH was a generous gift of Jean Rivier (Salk Institute, La Jolla, CA), and the CRH antagonist α-helical CRH residues 9–41 (αhCRH9–41) was obtained from Rivier and Peninsula Laboratories (Belmont, CA). CRH was dissolved in 0.9% NaCl, and αhCRH9–41 was dissolved in 0.1% BSA, pH 7.4 (adjusted with ammonium hydroxide vapor). CP-154,526, a CRH type 1 receptor antagonist, a generous gift from Pfizer Pharmaceuticals (Groton, CT), was dissolved in 0.9% NaCl (pH 4.2) (23). In all cases, solute dissolved completely in solvent, with no particulate matter remaining. Vehicle solutions lacking either CRH or CRH antagonists were used as controls.
Behavioral Tests.
All behavioral tests were conducted between 0900 and 1700. Each experiment contained 5–20 animals per group. Significance was determined by Student’s t test for comparison of two groups and by ANOVA for comparison of more than two groups. In restraint experiments, animals were tested immediately after a 30-min restraint in ventilated 50-ml polypropylene tubes, fitted so that animals could breathe but not move otherwise (24).
To study food-reinforced operant conditioning, animals were restricted to 3.5 g per day of standard chow one day prior to the conditioning procedure. Animals were placed on a fixed-ratio schedule requiring five responses to obtain one drop of 50% diluted condensed milk, with each session lasting approximately 60 min until the behavior was acquired. Several days after 100% acquisition, the rate at which the animals extinguished the behavior was assessed.
To assess pain sensitivity, latencies for the tail flick reflex were measured after a light was focused on the tail. As an additional measure, each mouse was placed on a metal surface heated to 56°C, and the latency of a characteristic hind-paw flick reflex was measured. For both tests, animals not responding within 5 s (tail flick) or 50 s (hot plate) were removed from the apparatus to avoid tissue damage.
For assessment of locomotor behavior, animals were placed in a well-lit open field (45 × 28 × 20 cm) for a 60-min observation period. Total distance traveled was assessed with the use of a tracking camera, while a photocell system recorded ambulatory photocell counts in 10-min intervals. The multicompartment chamber (MCC) has nine separate compartments, each with a hole in the floor and a wire stimulus just below the floor level. The mice lick, chew, or sniff the stimulus, and the mean time for which they do this (mean time per contact) is scored as the stress-sensitive measure (25, 26). The elevated-plus maze (EPM) consists of a black Plexiglas cross (arms 30 cm long × 5 cm wide) elevated 40 cm above the floor. Two of the arms (closed arms) are enclosed by 15 cm high transparent walls. Mice are placed in the center facing a closed arm and video taped for 5 min. The percentage of time spent in the open arms is scored (27). In the open-field, EPM, and MCC tests, the apparatuses were cleaned with 1% acetic acid to mask the odor of the preceding animals.
For the measurement of startle reflex responses, after a 5-min habituation period in a sound and light-attenuating startle chamber, sixty 50-ms air-puffs (15 psi), with various time intervals between them (25, 30, or 35 s) were delivered to the dorsal side of the mouse through a tube (0.4 cm i.d.) located 1 cm above the mouse. Startle stimuli and responses were controlled and recorded by using the SR-LAB startle response system (San Diego Instruments, San Diego). This system includes a clear acrylic cylinder (9.5 cm long, 3.2 cm i.d.) that is connected to an accelerometer, which transduces the mouse’s movement into voltage changes. Startle amplitude is defined as the average accelerometer voltage (Vavg) measured during a 200-ms recording window.
Conditioned-fear and shock-induced freezing trials were conducted in a shock-box apparatus (Lafayette Instruments, Lafayette, IN). All trials were video-monitored, and the percentage of time spent freezing (lack of all movement except that required for respiration) was subsequently scored. For the conditioned-fear study, animals were monitored for 6 min prior to receiving any shocks, and then immediately received a 1-ms shock every 10 s for 6 min. Twenty-four hours later, freezing behavior was observed for 6 min in a neutral cage of approximately the same size as the shock box. Animals were then immediately placed in the original shock box for a 6-min observation period without receiving any shocks. For the shock-induced freezing study, following a 6 min preshock observation period, animals received three 1-s shocks, each separated by 20 s. Freezing behavior was scored for 10 min after the shocks.
Surgery and Intracerebroventricular (i.c.v.) Injection.
For delivery of CRH, mice were anesthetized with sodium pentobarbital and implanted with polyethylene cannulae in the lateral ventricles as previously described (28). Animals were then singly housed, and 13–17 days later, i.c.v. infusions of vehicle (0.9% saline) or 20 ng of CRH (2 μl total volume) were slowly made into each lateral ventricle with an adapted Hamilton syringe (28).
For delivery of CRH antagonists, mice were anesthetized with 2.5% avertin, and guide cannulae (Plastics One, Roanoke, VA) were implanted into the right lateral ventricle (0.5 mm posterior, 1.4 mm lateral to bregma, and a depth of 2.5 mm) with a stereotaxic instrument (model 900; David Kopf Instruments, Tujunga, CA). After a 1-week recovery period, 4 μl of either vehicle (distilled H2O + 0.1% BSA) or αhCRH9–41 (25 μg = 6 nmol), or vehicle (saline, pH 4.2) or CP-154,526 (3.2 μg = 7 nmol), were slowly injected into the lateral ventricle with a 33-gauge internal cannula and a 10-μl Hamilton syringe. Eighteen minutes later, shock-induced freezing was assessed as described above. After the experiment, cannulae placements were confirmed by demonstrating successful injection of ink through the cannulae into the ventricular system.
In Situ Hybridization of mRNA.
After rapid decapitation of mice between 0800 and 1000, brains (n = 5 WT, 3 KO) were quickly frozen in isopentane cooled to −30°C on dry ice and stored at −80°C until they were sectioned. Cryostat (Leica, Deerfield, IL) sections 12 μm thick were thaw-mounted on SuperFrost Plus slides (Fisher). After air-drying at room temperature, sections were stored at −20°C with desiccant. For fixation, slide boxes were warmed to room temperature prior to opening. Slides were placed in phosphate-buffered 4% paraformaldehyde for 15 min, followed by 2 min in 2× SSC (0.75 M sodium chloride/0.075 M sodium citrate, pH 7.0), 2 min in 0.1 M triethanolamine, 10 min in 0.25% acetic anhydride in 0.1 M triethanolamine, 2 min in 2× SSC, progressively dehydrated in 50% to 100% ethanol, followed by 5 min in chloroform and 2 min in 95% ethanol. If not used immediately, slides were stored at −20°C with desiccant.
The urocortin cRNA probe was complementary to nucleotides 660–882 (29) of the 3′ coding and untranslated region of urocortin mRNA, and had 42% identity with the corresponding region of CRH mRNA (maximum length of identity, 6 nucleotides). The CRH cRNA probe was complementary to nucleotides 1517–2100 (30) of exon II of the mouse CRH gene (kindly provided by Audrey Seasholtz, Univ. of Michigan). This region of CRH mRNA has 43% identity with the corresponding region of urocortin mRNA (maximum length of identity, 14 nucleotides). This CRH probe had no cross-hybridization to urocortin mRNA, as it gave no signal in CRH KO mice, even in regions containing urocortin mRNA. Radiolabeled urocortin or CRH cRNA probes were synthesized by using the enzymes T3 or SP6, respectively, and [α-33P]UTP (specific activity 1,000–3,000 Ci/mmol, 10 mCi/ml, New England Nuclear; 1 mCi = 37 MBq). Once generated, probes (3 × 107 cpm/ml) were stored at −20°C in hybridization solution (50% deionized formamide/10% dextran sulfate/0.5 M NaCl/1× Denhardt’s solution/10 mM Tris, pH 8/1 mM EDTA/500 μg/ml yeast tRNA/10 mM DTT) until use.
For in situ hybridization, adjacent sections of each brain were examined for urocortin or CRH mRNA distribution. The probe in hybridization solution was heated to 65°C for 3 min, 100 μl of hybridization solution with probe was put on each slide, and then a coverslip was placed over the slide, avoiding air bubbles. Slides were placed in an air-tight chamber on top of filter paper saturated with 50% formamide/0.5 M NaCl and were hybridized for a minimum of 16 h at 60°C. Post-hybridization wash steps were carried out as follows. Coverslips were soaked off in 2× SSC at room temperature for 15 min, followed by another 15-min wash in 2× SSC at room temperature. RNase digestion was carried out for 30 min, at 37°C in a solution containing 20 μg/ml RNase A (Boehringer Mannheim), 0.5 M NaCl, 10 mM Tris, pH 8, and 1 mM EDTA. Next, the slides were washed in 2× SSC for 10 min at room temperature, followed by three 10-min washes in 0.5× SSC at room temperature. After washing for 30 min in 0.5× SSC at 65°C, slides were sequentially treated at room temperature with 0.5× SSC for 2 min, 50% ethanol/0.3 M ammonium acetate for 1 min, 70% ethanol/0.3 M ammonium acetate for 1 min, 95% ethanol/0.15 M ammonium acetate for 1 min, and finally, twice with 100% ethanol for 1 min each. Slides were allowed to air-dry, and then they were exposed to x-ray film (Hyperfilm βmax, Amersham) for 1–14 days.
Some of the slides of each genotype (n = 5 WT, 2 KO) were dipped in NBT-2 emulsion (Eastman Kodak) diluted 1:1 in distilled water for more precise anatomical localization. The slides were developed after 27 days exposure [4 min in D19 (Eastman Kodak) 1:1 in distilled water, 15 s in distilled water, and 5 min in Kodak fixer], and counterstained with 0.01% toluidine blue (Fisher). All slides were carefully analyzed for detection of signal above background.
RESULTS
Basal Behavior Is Normal in CRH-Deficient Mice.
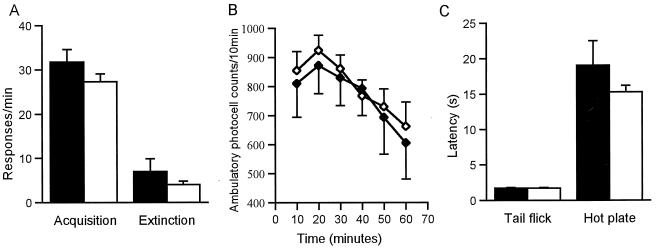
Mice with targeted deletion of the CRH gene exhibit normal growth, fertility, and longevity despite the complete absence of CRH and a blunted hypothalamic-pituitary-adrenal axis response to a variety of stressors (22). The learning ability of CRH KO mice appears to be normal, in that the animals acquire, perform, and extinguish a food-reinforced operant task at the same rate as do WT mice (Fig. 1A). The locomotor activity of CRH KO mice is similar to that of WT mice as assessed in an open field, both by ambulatory photocell counts and in total distance traveled (Fig. 1B). Pain responses appear normal in CRH KO mice, as their behavior is indistinguishable from that of WT mice in both the tail flick and the hot plate assays (Fig. 1C).
Figure 1.
Basal learning, locomotor activity, and pain sensitivity are normal in CRH KO mice. (A) Food-reinforced operant task as a measure of learning ability. CRH KO mice learn the task within the same time as WT animals (55.6 vs. 54 h at 100% acquisition, WT vs. KO). At acquisition (A, acquisition), responses/min are not different between WT and KO mice. At extinction (A, extinction) responses/min are again not different between WT and KO mice. (B) Open field. CRH KO mice have normal activity in an open field by ambulatory photocell counts. The total distance traveled was also similar between WT and KO mice (13,243 ± 2,145 vs. 13,484 ± 1,346 cm, WT vs. KO). (C) Pain sensitivity is normal in CRH KO mice, measured in both tail flick and hot plate assays. In A–C, WT, closed symbols; CRH KO, open symbols. Error bars indicate SEM.
Behavioral Responses to Stressors Are Normal in CRH-Deficient Mice.
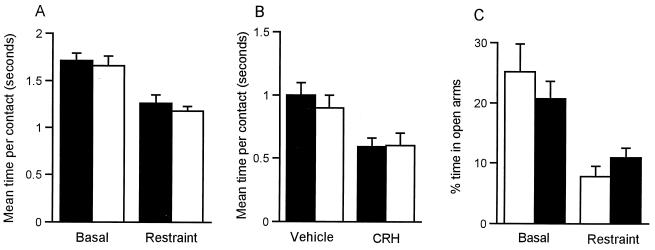
As an initial measure of the behavioral response to stressors of CRH KO mice, animals were observed in 25-min trials in the MCC. In the MCC, the mean time per contact with a stimulus object is decreased by prior stressful treatments or i.c.v. CRH (26). The behavior of unrestrained CRH KO and WT mice did not differ significantly (Fig. 2A). After 30 min of restraint, both WT and CRH KO mice significantly decreased their mean time per contact to the same degree (P < 0.005) (Fig. 2A). In addition, CRH KO mice responded to i.c.v. administration of 20 ng of CRH indistinguishably from WT mice, with both genotypes significantly decreasing their mean time per contact after infusion of CRH (P < 0.02) (Fig. 2B). The normal response of CRH KO mice to i.c.v. administration of CRH indicates that the CRH receptor system is intact, and that there is no compensatory up-regulation of CRH receptor sensitivity due to the CRH deficiency. The EPM, which exploits a rodent’s natural preference for enclosed spaces, was used as another measure of anxiety-related behavior. Decreased time spent in the open arms of the maze is thought to correlate with increased anxiety (27). The time spent in the open arms of the EPM at baseline was similar in WT and KO mice, and both genotypes significantly reduced their time spent in the open arms after 30 min of restraint to a similar extent (P < 0.01) (Fig. 2C). In both the MCC and the EPM, one consistent difference between WT and CRH-deficient mice was that KO mice exhibited significantly increased grooming behavior (data not shown).
Figure 2.
CRH KO mice have normal behavioral responses to stress and to i.c.v. CRH. (A and B) MCC. Basal activity (time per contact) of CRH KO and WT mice is equivalent (A, Basal) and decreases to a similar extent in both genotypes after a 30-min restraint (A, Restraint). Activity after i.c.v. injection of vehicle is similar in WT and CRH KO mice (B, Vehicle) and decreases significantly to the same extent in both genotypes after i.c.v. injection of CRH (B, CRH). (C) EPM. Basally, the time spent in the open arms was equivalent in WT and CRH KO (C, Basal), and it significantly decreases in both genotypes to the same degree after a 30-min restraint (C, Restraint). In A–C, WT, closed bars; CRH KO, open bars.
CRH-deficient mice were also analyzed in several other paradigms thought to reflect fear or anxiety. The startle response to a tactile stimulus (15-psi air-puff) did not differ significantly between KO and WT mice (Fig. 3A). In a conditioned-fear model, animals were monitored for 6 min in a shock-box apparatus (Fig. 3B, Pre) before administration of foot shocks (1-ms shock every 10 s for 6 min). Twenty-four hours after the shock paradigm, mice were first observed for 6 min in a neutral cage, and neither genotype had a significant increase in freezing (Fig. 3B, Neutral). However, when mice were next immediately placed in the original shock box for a 6-min observation without receiving any shocks, both genotypes significantly increased freezing upon this reexposure to the shock environment (P < 0.00001) (Fig. 3B, Post). The responses of KO and WT mice did not differ significantly from each other during any of the three observation periods.
Figure 3.
CRH KO mice exhibit normal startle and fear-conditioned responses. (A) The average accelerometer voltage of the startle response to repeated puffs of air is indistinguishable between WT and CRH KO mice. (B) Fear-conditioned freezing. The two genotypes exhibit freezing behavior for similar amounts of time before application of foot shock (Pre). Twenty-four h after 36 foot shocks (100-ms duration every 10 s), WT and CRH KO mice have no increase in freezing in a neutral chamber (Neutral) but exhibit a similar, significant 7-fold increase in conditioned freezing when placed in the original shock chamber (Post). In A–B, WT, closed symbols; CRH KO, open symbols.
CRH Antagonists Block Stress-Induced Behavior in CRH-Deficient Mice.
To ascertain whether a molecule other than CRH can interact with CRH receptors to mediate stress-induced behavior, the effects of two chemically unrelated CRH antagonists were tested in WT and KO mice in a second shock-induced freezing paradigm (Fig. 4). In this paradigm, mice received three 1-s shocks, each separated by 20 s, followed immediately by quantitation of shock-induced freezing. Eighteen minutes prior to the shocks, animals had received i.c.v. infusions of vehicle, the peptide antagonist, αhCRH9–41 (6 nmol), or the nonpeptide CRH type 1 receptor antagonist, CP-154,526 (7 nmol). CP-154,526 has >1000-fold selectivity for the type 1 receptor (31). In a 6-min preshock observation period, infusion of the antagonists did not significantly affect the behavior of either genotype as compared with the vehicle controls, with all groups freezing less than 6.5% of the time. Immediately after the shock paradigm, there was a marked increase in freezing behavior in the WT and KO mice that had received vehicle infusions, with no significant difference in the amount of freezing between either the two genotypes or between the two vehicles (Fig. 4).
Figure 4.
CRH antagonists (A, α-helical CRH; B, CP-154,526) block shock-induced freezing to the same degree in both WT and CRH KO mice. After i.c.v. administration of vehicle followed by 3 foot shocks (1-s duration every 20 s), the two genotypes exhibit similar amounts of freezing (A, Vehicle; B, Vehicle). i.c.v. administration of two chemically unrelated CRH antagonists, either the nonselective α-helical CRH9–41 (A, alpha-helical CRH) or the CRH type I receptor-specific CP-154,526 (B, CP-154,526) significantly blocks shock-induced freezing to the same degree in WT and CRH KO mice. In A–B, WT, closed bars; CRH KO, open bars.
However, as compared with vehicle controls, both αhCRH9–41 and CP-154,526 significantly decreased freezing time in the postshock period in both WT and KO mice (Fig. 4 A and B). Within and between genotypes, αhCRH9–41 and CP-154,526 were equally effective in inhibiting shock-induced freezing. As the only similarity between these two chemically unrelated compounds (32, 33) is their ability to block the biological effects of CRH and CRH-like peptides (21, 31, 34), their observed attenuation of stressful behavior is most likely due to specific blockade of CRH receptors rather than to nonspecific actions.
Urocortin mRNA Distribution Is Normal in CRH-Deficient Mouse Brain.
Since urocortin can bind to type 1 CRH receptors (20, 35), its blockade by CRH antagonists might conceivably explain the actions of CP-154,526 and αhCRH9–41 in CRH KO mice. For example, urocortin expression might be altered in CRH-deficient animals to compensate for their lack of CRH, or it might mediate stress-induced behaviors in normal mice as well, possibly in concert with CRH. To address these possibilities, we examined in WT and CRH KO brains the distribution of urocortin and CRH mRNA expression by using in situ hybridization to 33P-labeled urocortin and CRH riboprobes. In our studies of shock-induced freezing, freezing behavior occurred within 1 min of a mouse’s being shocked. As the content of mRNA encoding a factor mediating this behavior was very unlikely to change during this short time period, we examined mRNA expression of x-ray film images in basal, unstressed mice. In WT mice, CRH mRNA was expressed at relatively high levels in the paraventricular nucleus of the hypothalamus (PVH), amygdala, olfactory bulb, cerebral cortex, inferior olive, and the medial preoptic nucleus (Fig. 5A and data not shown). Lower levels were detected in the entopeduncular nucleus, the vestibular spinal nucleus, and the subthalamic nucleus (data not shown). In these same brains, urocortin mRNA was expressed only in the area of the Edinger–Westphal nucleus, including the adjacent nucleus of Darkschewitsch (Fig. 5B). Specifically, urocortin mRNA was absent in the lateral septum, hippocampus, substantia nigra, and hypothalamus, as well as in the amygdala and other brain regions thought to mediate stress-related behaviors (Fig. 5C). Whereas no hybridization to the CRH riboprobe was detected in CRH KO brain (Fig. 5D), the distribution of urocortin mRNA expression in CRH KO mice was identical to that of WT mice, being confined to the area of the Edinger–Westphal nucleus and nucleus of Darkschewitsch (Fig. 5E). Specifically, there was no ectopic urocortin mRNA expression in CRH KO brain in areas that normally express CRH, such as the PVH or amygdala (Fig. 5F).
Figure 5.
Urocortin mRNA expression is confined to the region of the Edinger–Westphal nucleus in both WT and CRH KO mouse brain. In WT animals, CRH mRNA expression (A) is clearly detectable in the PVH (vertical arrow) and amygdala (horizontal arrows). Urocortin mRNA expression is detectable in the Edinger–Westphal nucleus (B, horizontal arrow), but not in the hypothalamus or amygdala (C). In CRH KO mice, no CRH expression is detected (D), whereas the presence of urocortin mRNA in the Edinger–Westphal nucleus (E) and its absence in hypothalamus (F) are identical to those of the WT. Brain sections in C and F are adjacent to those in A and D, respectively, and thus contain the paraventricular nucleus and amygdala.
Because a much wider expression of urocortin mRNA in brain had been reported previously (20, 36), we sought to ensure that our in situ hybridization method was both sensitive and specific. We therefore examined adjacent emulsion-dipped slides from WT and CRH KO brain for both CRH and urocortin mRNA expression (Fig. 6). In WT mice, the pattern of CRH mRNA distribution confirmed adequate sensitivity, with expression being readily detected in the PVH (Fig. 6A), amygdala (Fig. 6C), and other areas detected by x-ray film that have been previously described to contain CRH mRNA (37). Background levels of radioactivity were defined by the pattern of emulsion autoradiography detected with the CRH probe in CRH KO brain (Fig. 6 B and D). Using these levels of CRH riboprobe autoradiography in WT and CRH KO brain as a guide, we detected urocortin mRNA by emulsion autoradiography only in the area of the Edinger–Westphal nucleus, with signal specifically absent in the PVH (Fig. 6 E and F), amygdala (Fig. 6 G and H), and all other brain regions of both genotypes (data not shown).
Figure 6.
Urocortin mRNA expression is not detectable in the PVH or amygdala of WT and KO mouse brain. In the PVH, robust CRH expression is detectable in WT (A) but not KO (B) mice. Likewise, in the amygdala, CRH is detected in WT (C) but not KO (D) animals. No urocortin expression is detectable in the PVH of either WT (E) or KO (F) animals, nor is it detectable in the amygdala of WT (G) or KO (H) animals. A and C are from the same brain section, and E and G are from an adjacent section. Similarly, B and D are from the same section, and this section is adjacent to the section shown in F and H. All sections are at same magnification, with bar = 100 μm. < and > delineate third ventricle.
DISCUSSION
CRH-deficient mice display normal behavior in multiple categories, including learning, locomotion, startle responsiveness, and pain sensitivity. They exhibit a normal increase in anxiety-like behavior after restraint or CRH administration into the brain. CRH KO and WT mice are also indistinguishable in their acquisition and retention of freezing behavior after exposure to foot shock. Freezing behavior immediately after foot-shock exposure is blocked to the same extent in normal and CRH KO mice by the prior i.c.v. administration of the CRH antagonists, αhCRH9–41 and CP-154,526. Because CP-154,526 and αhCRH9–41 can also block urocortin (21, 38) and thereby possibly explain the antagonists’ effects in CRH KO mice, we examined the distribution of urocortin and CRH mRNAs in normal and CRH KO mouse brain. CRH mRNA expression was confirmed in previously described regions of the normal mouse brain (37), and was, as expected, absent from CRH KO brain. In both genotypes, urocortin mRNA expression was confined to the region of the Edinger–Westphal nucleus and was absent from regions known to mediate stress-responsive behaviors. Specifically, there was no ectopic urocortin mRNA expression in CRH KO brain in areas that normally express CRH, such as the amygdala or the hypothalamic paraventricular nucleus.
Our studies indicate a role for the CRH type I receptor in anxiety-related behaviors, as we were able to attenuate shock-induced freezing with CP-154,526. While αhCRH9–41 can block both the type 1 and type 2 CRH receptors, the selectivity of CP-154,526 for the type 1 CRH receptor (31) suggests that this receptor mediates the behavioral response to stressors. This conclusion is supported by studies of CRH type 1 receptor knockout mice in which these mice show behavior consistent with decreased anxiety (16, 17). In addition, using antisense oligonucleotide treatment, knocking down CRH type 1, but not type 2 receptor expression, produces a significant anxiolytic effect in defensive withdrawal (39). Similar to our studies in mice, CP-154,526, also known as antalarmin (40), attenuates shock-induced freezing and conditioned fear responses in rats (18).
Urocortin can bind to and activate both type 1 and type 2 CRH receptors with a higher affinity than does CRH (20). Central infusion of urocortin decreases appetite (41), causes anxiety-like behavior as measured in the dark–light emergence task and the EPM, and decreases exploratory behavior in an open field (21). In addition, the anxiogenic effects of centrally infused urocortin can be blocked by coadministration of αhCRH9–41 (21). To address the possibility that an altered pattern of expression of urocortin might compensate for the lack of CRH in CRH KO animals, or that it might mediate part of the behavioral response to stress in WT animals, and thus might be expressed in regions of WT brain known to be involved in anxiety-related behaviors, we mapped urocortin mRNA expression by in situ hybridization in WT and CRH-deficient animals. As positive and negative controls for this study, we also mapped CRH mRNA expression in WT and CRH KO mice. In our studies of shock-induced freezing, the freezing behavior blocked by CRH antagonists occurred within 1 min of the stimulus, and thus must have been mediated by a preformed pool of peptide translated from mRNA antedating the stimulus. Thus, we examined mRNA expression patterns in animals under basal conditions.
CRH expression was detected in the expected brain regions of WT animals (37), including high levels in the amygdala and PVH, whereas no signal was detected in CRH KO animals. In both genotypes, urocortin expression was detected exclusively in the Edinger–Westphal nucleus and the adjacent nucleus of Darkschewitsch. This limited expression of urocortin is at odds with several other studies that have employed either in situ hybridization to urocortin mRNA or immunocytochemistry for urocortin peptide. However, except for expression in the region of the Edinger–Westphal nucleus, there is very little agreement among these other studies. For example, using in situ hybridization, Wong et al. (36) reported widespread urocortin mRNA expression throughout the brain, whereas Vaughan et al.(20) detected urocortin mRNA in a much more limited distribution outside of the Edinger–Westphal nucleus. Neither report of mRNA distribution is in agreement with two immunohistochemistry studies in which urocortin peptide expression outside of the Edinger–Westphal and nucleus of Darkschewitsch has been reported either in the paraventricular nucleus, supraoptic nucleus, ventral medial nucleus, periventricular nucleus, the raphe nucleus, interstitial nucleus of Cajal, the red nucleus, the substantia nigra, and the lateral septum (42), or in substantia nigra and the ventral tegmental area (43). The lack of agreement between our studies and those of others is puzzling, but is likely because of differences in the specificity or sensitivity of the in situ hybridization methods. We took advantage of the known distribution of CRH mRNA in mouse brain (37) and the availability of the CRH KO mouse (22) to assess the sensitivity and specificity, respectively, of our in situ hybridization method. Although the lengths (and therefore the specific radioactivity per molecule) of the CRH and urocortin probes differed, under the conditions that we found specific hybridization signals for CRH mRNA in WT (Figs. 5A and 6 A and C) and for urocortin mRNA in WT and KO (Figs. 5 B and E), the levels of background hybridization were similar with the two probes (compare Fig. 6 B, D, E–H).
In our study we did not find any urocortin expression outside of the region of the Edinger–Westphal nucleus in either WT or CRH KO mice. The Edinger–Westphal nucleus is not known to project to any brain regions suggested to play a role in anxiety-like behavior (44–46). Anterograde tracing studies in the rat (44) or cat (45, 46) have demonstrated two descending projections from the Edinger–Westphal nucleus in addition to projections to the dendrites of the ciliary ganglion cells. These two descending pathways project to the olivary nuclei, lateral parabrachial nucleus, facial nucleus, trigeminal brainstem nuclear complex, reticular nucleus, and the sympathetic and parasympathetic preganglionic cell columns and lamina I of the spinal cord. All of the brainstem nuclei mentioned have CRH type I receptor mRNA (13). These data suggest that the Edinger–Westphal nucleus may regulate some aspects of autonomic nervous system function, as well as sensory inputs, including pain. Although the Edinger–Westphal nucleus might thus play a role in mediating stress-induced behaviors through an indirect pathway involving the autonomic nervous system, none of these brain regions have been implicated in the behavioral response to stress. Therefore, an entirely novel behavioral pathway must be postulated to accommodate the possibility that urocortin in the Edinger–Westphal nucleus mediates stress-induced behaviors in the CRH KO.
If such a behavioral pathway involving urocortin in the Edinger–Westphal nucleus exists in the CRH KO, it is highly unlikely to be confined to that genotype, with stress-induced behaviors in WT animals acting through traditional pathways involving CRH in the amygdala (47, 48). As we find no difference in behavioral responses to stress in WT and KO animals, urocortin in the Edinger–Westphal nucleus of CRH KO mice would have to compensate for the lack of CRH through an indirect pathway to induce behaviors that perfectly mimic those seen in WT animals. Therefore, if urocortin mediates stress-induced behaviors in CRH-deficient mice through descending projections from the Edinger–Westphal nucleus affecting the autonomic nervous system, it seems likely that this pathway also mediates stress-induced behaviors in WT animals. On the other hand, if urocortin is not involved in mediating behavior in either genotype through such novel mechanisms, then our studies suggest that an unidentified CRH-like molecule may participate in stress-induced behaviors.
Acknowledgments
We thank Jean Rivier and Pfizer Pharmaceuticals for the generous gifts of αhCRH9–41 and CP-154,526, respectively. We also thank Allison Carrigan and Chris Lage for animal husbandry, Barry Kosofsky for the use of equipment, Peter Lio and Michael Adamkiewicz for technical assistance, and the members of the Majzoub and Endocrine research laboratories, especially Frederick Grant, for helpful discussions. This work was supported in part by grants from the Howard Hughes Medical Institute (S.W., L.M.), National Institutes of Health (J.M., L.M., A.D., K.M., C.B., A.S.), and the National Institute of Mental Health (A.D.), and by a Burroughs Wellcome Fund Career Development Award (L.M.).
ABBREVIATIONS
- CRH
corticotropin-releasing hormone
- αhCRH9–41
α-helical CRH residues 9–41
- KO
knockout: WT, wild-type
- MCC
multicompartment chamber
- EPM
elevated-plus maze
- i.c.v.
intracerebroventricular
- PVH
paraventricular nucleus of the hypothalamus
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Selye H. J Human Stress. 1975;1:37–44. doi: 10.1080/0097840X.1975.9940406. [DOI] [PubMed] [Google Scholar]
- 2.Checkley S. Br Med Bull. 1996;52:597–617. doi: 10.1093/oxfordjournals.bmb.a011570. [DOI] [PubMed] [Google Scholar]
- 3.Gold P, Goodwin F, Chrousos G. N Engl J Med. 1988;319:348–353. doi: 10.1056/NEJM198808113190606. [DOI] [PubMed] [Google Scholar]
- 4.Gold P W, Wong M L, Chrousos G P, Licinio J. Mol Psychiatry. 1996;1:257–264. [PubMed] [Google Scholar]
- 5.Owens M J, Nemeroff C B. Ciba Found Symp. 1993;172:296–308. doi: 10.1002/9780470514368.ch15. [DOI] [PubMed] [Google Scholar]
- 6.Vale W, Spiess J, Rivier C, Rivier J. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 7.Sutton R, Koob G, Le Moal M, Rivier J, Vale W. Nature (London) 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- 8.Britton K, Lee G, Vale W, Rivier J, Koob G. Brain Res. 1986;369:303–306. doi: 10.1016/0006-8993(86)90539-1. [DOI] [PubMed] [Google Scholar]
- 9.Dunn A, Berridge C. Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 10.Heinrichs S, Menzaghi F, Pich E, Britton K, Koob G. Ann N Y Acad Sci. 1995;771:92–104. doi: 10.1111/j.1749-6632.1995.tb44673.x. [DOI] [PubMed] [Google Scholar]
- 11.Stenzel-Poore M, Heinrichs S, Rivest S, Koob G, Vale W. J Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dieterich K D, Lehnert H, De Souza E B. Exp Clin Endocrinol Diabetes. 1997;105:65–82. doi: 10.1055/s-0029-1211730. [DOI] [PubMed] [Google Scholar]
- 13.Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko P, Vale W. Proc Natl Acad Sci USA. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovenberg T, Liaw C, Grigoriadis D, Clevenger W, Chalmers D, De Souza E, Oltersdorf T. Proc Natl Acad Sci USA. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lovenberg T, Chalmers D, Liu C, De Souza E. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- 16.Smith G W, Aubry J M, Dellu F, Contarino A, Bilezikjian L, Gold L, Chen R, Marchuk Y, Hauser C, Bentley C, et al. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 17.Timpl P, Spanagel R, Sillaber I, Kreese A, Reul J, Stalla G K, Blanquet V, Steckler T, Holsboer F, Wurth G. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 18.Deak T, Nguyen K T, Ehrlich A L, Watkins L R, Spencer R L, Maier S F, Licinio J, Wong M L, Chrousos G P, Webster E, Gold P W. Endocrinology. 1999;140:79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- 19.Chrousos G P, Gold P W. J Am Med Assoc. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 20.Vaughan J, Donaldson C, Bittencort J, Perrin M, Lewis K, Sutton S, Chan R, Turnbull A, Lovejoy D, Rivier C, et al. Nature (London) 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 21.Moreau J L, Kilpatrick G, Jenck F. NeuroReport. 1997;8:1697–1701. doi: 10.1097/00001756-199705060-00027. [DOI] [PubMed] [Google Scholar]
- 22.Muglia L, Jacobson L, Dikkes P, Majzoub J A. Nature (London) 1995;373:427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- 23.Guanowsky V, Chen Y L, Seymour P. Soc Neurosci Abstr. 1997;23:522. [Google Scholar]
- 24.Jeong K H, Jacobson L, Widmaier E P, Majzoub J. Endocrinology. 1999;140:1702–1708. doi: 10.1210/endo.140.4.6669. [DOI] [PubMed] [Google Scholar]
- 25.Arnsten A T, Segal D S. Life Sci. 1979;25:1035–1042. doi: 10.1016/0024-3205(79)90589-7. [DOI] [PubMed] [Google Scholar]
- 26.Berridge C W, Dunn A J. Regul Pept. 1986;16:83–93. doi: 10.1016/0167-0115(86)90196-5. [DOI] [PubMed] [Google Scholar]
- 27.Pellow S, Chopin P, File S E, Briley M. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 28.Guild A L, Dunn A J. Pharmacol Biochem Behav. 1982;17:31–36. doi: 10.1016/0091-3057(82)90258-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhao L, Donaldson C, Smith G, Vale W W. Genomics. 1998;50:23–33. doi: 10.1006/geno.1998.5292. [DOI] [PubMed] [Google Scholar]
- 30.Seasholtz A, Bourbonais F J, Harnden C E, Camper S A. Mol Cell Neurosci. 1991;2:266–273. doi: 10.1016/1044-7431(91)90054-r. [DOI] [PubMed] [Google Scholar]
- 31.Schulz D W, Mansbach R S, Sprouse J, Braselton J P, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt A W, Seeger T, et al. Proc Natl Acad Sci USA. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y L, Mansbach R S, Winter S M, Brooks E, Collins J, Corman M L, Dunaiskis A R, Faraci W S, Gallaschun R J, Schmidt A, Schulz D W. J Med Chem. 1997;40:1749–1754. doi: 10.1021/jm960861b. [DOI] [PubMed] [Google Scholar]
- 33.Rivier J, Rivier C, Vale W. Science. 1984;224:889–891. doi: 10.1126/science.6326264. [DOI] [PubMed] [Google Scholar]
- 34.Olianas M, Lampis G, Onali P. J Neurochem. 1995;64:394–401. doi: 10.1046/j.1471-4159.1995.64010394.x. [DOI] [PubMed] [Google Scholar]
- 35.Behan D P, Grigoriadis D E, Lovenberg T, Chalmers D, Heinrichs S, Liaw C, De Souza E B. Mol Psychiatry. 1996;1:265–277. [PubMed] [Google Scholar]
- 36.Wong M L, al-Shekhlee A, Bongiorno P B, Esposito A, Khatri P, Sternberg E M, Gold P W, Licinio J. Mol Psychiatry. 1996;1:307–312. [PubMed] [Google Scholar]
- 37.Keegan C E, Herman J P, Karolyi I J, O’Shea K S, Camper S A, Seasholtz A F. Endocrinology. 1994;134:2547–2555. doi: 10.1210/endo.134.6.8194481. [DOI] [PubMed] [Google Scholar]
- 38.Baram T Z, Chalmers D T, Chen C, Koutsoukos Y, De Souza E B. Brain Res. 1997;770:89–95. doi: 10.1016/s0006-8993(97)00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heinrichs S C, Lapsansky J, Lovenberg T W, De Souza E B, Chalmers D T. Regul Pept. 1997;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- 40.Webster E L, Lewis D B, Torpy D J, Zachman E K, Rice K C, Chrousos G P. Endocrinology. 1996;137:5747–5750. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- 41.Spina M, Merlo-Pich E, Chan R K, Basso A M, Rivier J, Vale W, Koob G F. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- 42.Kozicz T, Yanaihara H, Arimura A. J Comp Neurol. 1998;391:1–10. doi: 10.1002/(sici)1096-9861(19980202)391:1<1::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto H, Maeda T, Fujimura M, Fujimiya M. Neurosci Lett. 1998;243:21–24. doi: 10.1016/s0304-3940(98)00071-8. [DOI] [PubMed] [Google Scholar]
- 44.Klooster J, Beckers H J, Vrensen G F, van der Want J J. Brain Res. 1993;632:260–273. doi: 10.1016/0006-8993(93)91161-k. [DOI] [PubMed] [Google Scholar]
- 45.Loewy A D, Saper C B. Brain Res. 1978;150:1–27. doi: 10.1016/0006-8993(78)90650-9. [DOI] [PubMed] [Google Scholar]
- 46.Loewy A D, Saper C B, Yamodis N D. Brain Res. 1978;141:153–159. doi: 10.1016/0006-8993(78)90624-8. [DOI] [PubMed] [Google Scholar]
- 47.Gray T S, Bingaman E W. Crit Rev Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- 48.Swiergiel A, Takahashi L, Kalin N. Brain Res. 1993;623:229–234. doi: 10.1016/0006-8993(93)91432-r. [DOI] [PubMed] [Google Scholar]