Abstract
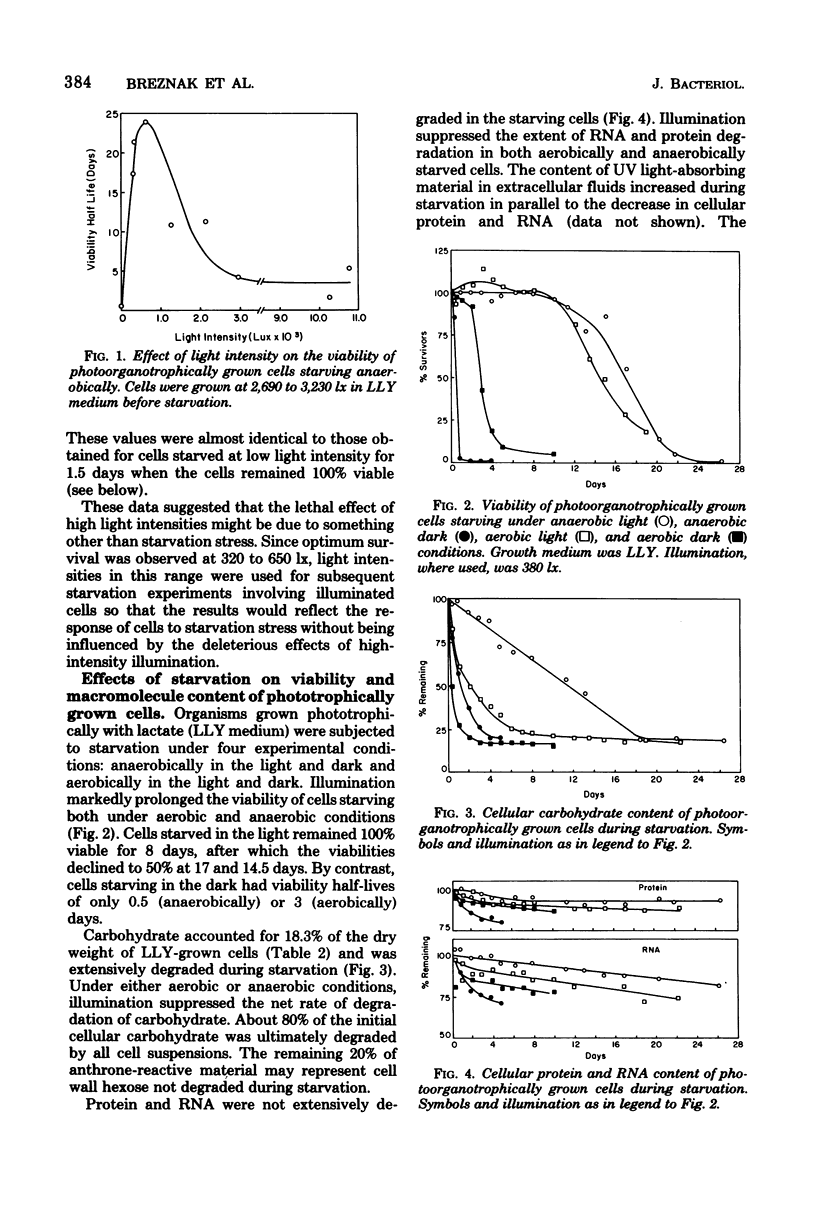
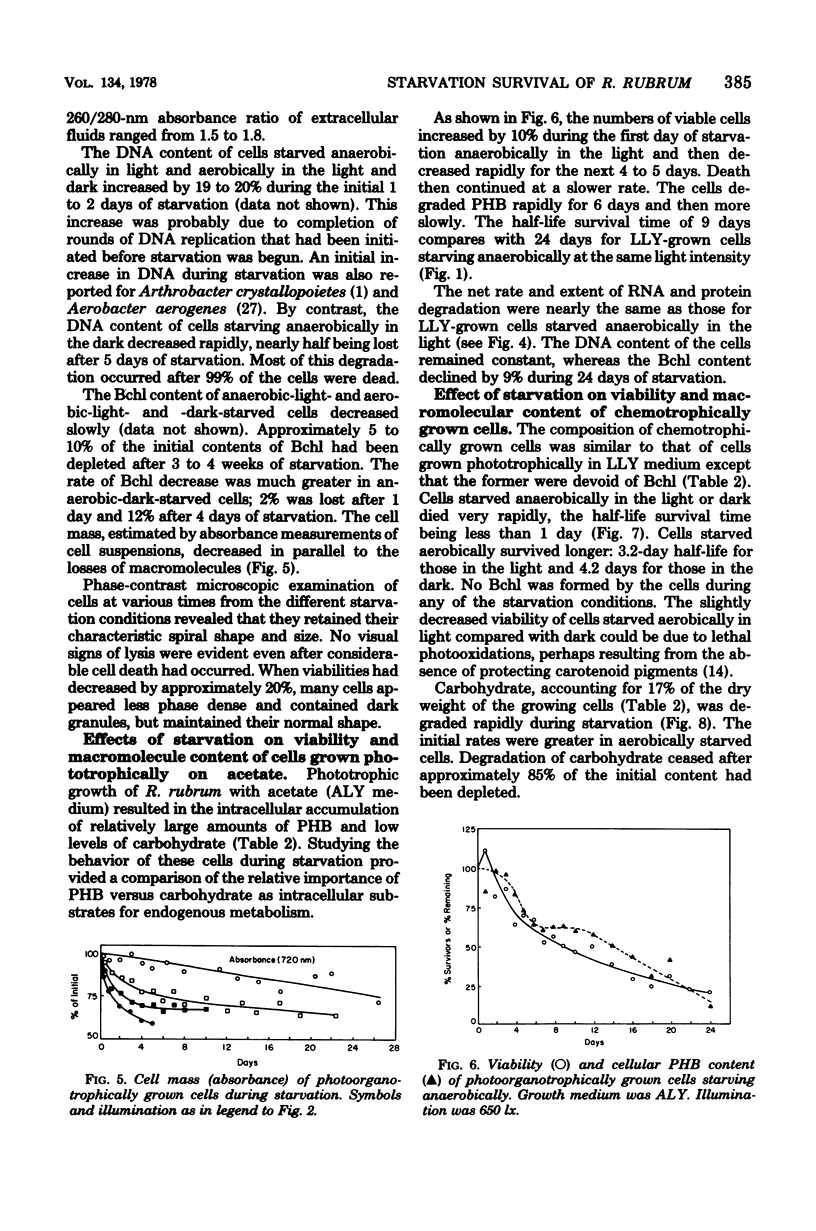
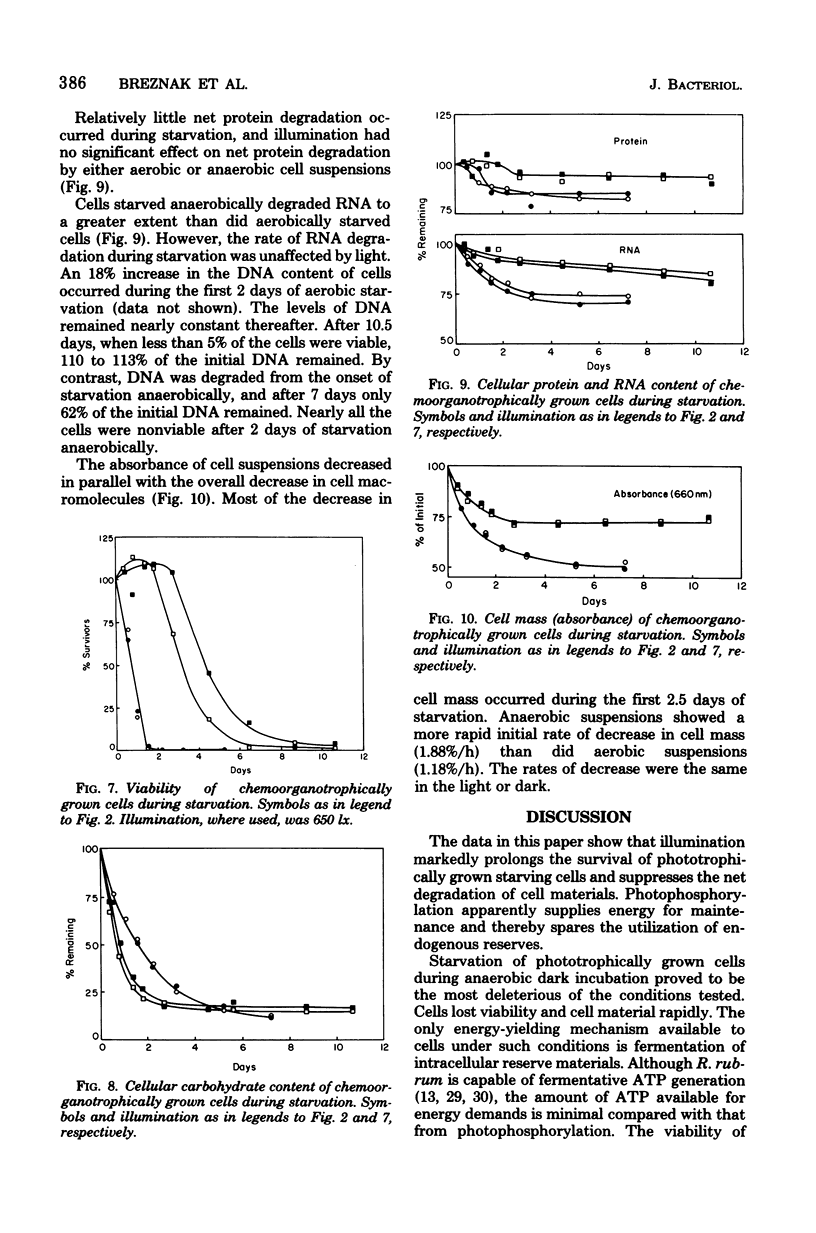
Cells of Rhodospirillum rubrum were grown photoorganotrophically and chemoorganotrophically and then starved for organic carbon and combined nitrogen under four conditions: anaerobically in the light and dark and aerobically in the light and dark. Illumination prolonged viability and suppressed the net degradation of cell material of phototrophically grown cells, but had no effect on chemotrophically grown cells that did not contain bacteriochlorophyll. The half-life survival times of carbohydrate-rich phototrophically grown cells during starvation anaerobically or aerobically in the light were 17 and 14.5 days, respectively. The values for starvation aerobically and anaerobically in the dark were 3 and 0.5 days, respectively. Chemotrophically grown cells had half-life survival times of 3 and 4 days during starvation aerobically in the light and dark, respectively, and 0.8 day during starvation anaerobically in the light or dark. Of all cell constituents examined, carbohydrate was most extensively degraded during starvation, although the rate of degradation was slowest for phototrophically grown cells starved anaerobically in the light. Phototrophically grown cells containing poly-β-hydroxybutyrate as carbon reserve were less able to survive starvation anaerobically in the light than were carbohydrate-rich cells starved under comparable conditions. Light intensity had a significant effect on viability of phototrophically grown cells starving anaerobically. At light intensities of 320 to 650 lx, the half-life survival times were 17 to 24 days. At 2,950 to 10,500 lx, the survival times decreased to 1.5 to 5.5 days. The kinetics of cell death correlated well with the rate of loss of cell mass of starving cells. However, the cause of death could not be attributed to degradation of any specific cell component.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boylen C. W., Ensign J. C. Intracellular substrates for endogenous metabolism during long-term starvation of rod and spherical cells of Arthrobacter crystallopoietes. J Bacteriol. 1970 Sep;103(3):578–587. doi: 10.1128/jb.103.3.578-587.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. K. TOWARD THE ISOLATION OF A PHOTOCHEMICAL REACTION CENTER IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1963 Nov 29;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. SOME ASPECTS OF THE ENDOGENOUS METABOLISM OF BACTERIA. Bacteriol Rev. 1964 Jun;28:126–149. doi: 10.1128/br.28.2.126-149.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. STUDIES ON THE ENDOGENOUS METABOLISM OF ESCHERICHIA COLI. Biochem J. 1965 May;95:332–343. doi: 10.1042/bj0950332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. The endogenous metabolism of microorganisms. Annu Rev Microbiol. 1962;16:241–264. doi: 10.1146/annurev.mi.16.100162.001325. [DOI] [PubMed] [Google Scholar]
- Ensign J. C. Long-term starvation survival of rod and spherical cells of Arthrobacter crystallopoietes. J Bacteriol. 1970 Sep;103(3):569–577. doi: 10.1128/jb.103.3.569-577.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLT S. C., MARR A. G. EFFECT OF LIGHT INTENSITY ON THE FORMATION OF INTRACYTOPLASMIC MEMBRANE IN RHODOSPIRILLUM RUBRUM. J Bacteriol. 1965 May;89:1421–1429. doi: 10.1128/jb.89.5.1421-1429.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempfling W. P., Mainzer S. E. Effects of varying the carbon source limiting growth on yield and maintenance characteristics of Escherichia coli in continuous culture. J Bacteriol. 1975 Sep;123(3):1076–1087. doi: 10.1128/jb.123.3.1076-1087.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., Thomashow M. F., Rittenberg S. C. Changes in cell composition and viability of Bdellovibrio bacteriovorus during starvation. Arch Microbiol. 1974 May 20;97(4):313–327. doi: 10.1007/BF00403070. [DOI] [PubMed] [Google Scholar]
- KOHLMILLER E. F., Jr, GEST H. A comparative study of the light and dark fermentations of organic acids by Rhodo-spirillum rubrum. J Bacteriol. 1951 Mar;61(3):269–282. doi: 10.1128/jb.61.3.269-282.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALLETTE M. F. Validity of the concept of energy of maintenance. Ann N Y Acad Sci. 1963 Jan 21;102:521–535. doi: 10.1111/j.1749-6632.1963.tb13658.x. [DOI] [PubMed] [Google Scholar]
- McGrew S. B., Mallette M. F. ENERGY OF MAINTENANCE IN ESCHERICHIA COLI. J Bacteriol. 1962 Apr;83(4):844–850. doi: 10.1128/jb.83.4.844-850.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze J., Kamen M. D. Adenosine triphosphate cellular levels in Rhodosopirillum rubrum during transition from aerobic to anaerobic metabolism. Biochim Biophys Acta. 1971 Apr 6;234(1):137–143. doi: 10.1016/0005-2728(71)90138-1. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- Pirt S. J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., WADE H. E., NESS A. G. The catabolism of proteins and nucleic acids in starved Aerobacter aerogenes. Biochem J. 1963 Feb;86:197–203. doi: 10.1042/bj0860197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Doudoroff M., Kunisawa R., Contopoulou R. THE ROLE OF ORGANIC SUBSTRATES IN BACTERIAL PHOTOSYNTHESIS. Proc Natl Acad Sci U S A. 1959 Aug;45(8):1246–1260. doi: 10.1073/pnas.45.8.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thore A., Keister D. L., San Pietro A. Studies on the respiratory system of aerobically (dark) and anaerobically (light) grown Rhodospirillum rubrum. Arch Mikrobiol. 1969;67(4):378–396. doi: 10.1007/BF00412584. [DOI] [PubMed] [Google Scholar]
- Uffen R. L. Growth properties of Rhodospirillum rubrum mutants and fermentation of pyruvate in anaerobic, dart conditions. J Bacteriol. 1973 Nov;116(2):874–884. doi: 10.1128/jb.116.2.874-884.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L., Wolfe R. S. Anaerobic growth of purple nonsulfur bacteria under dark conditions. J Bacteriol. 1970 Oct;104(1):462–472. doi: 10.1128/jb.104.1.462-472.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



