Abstract
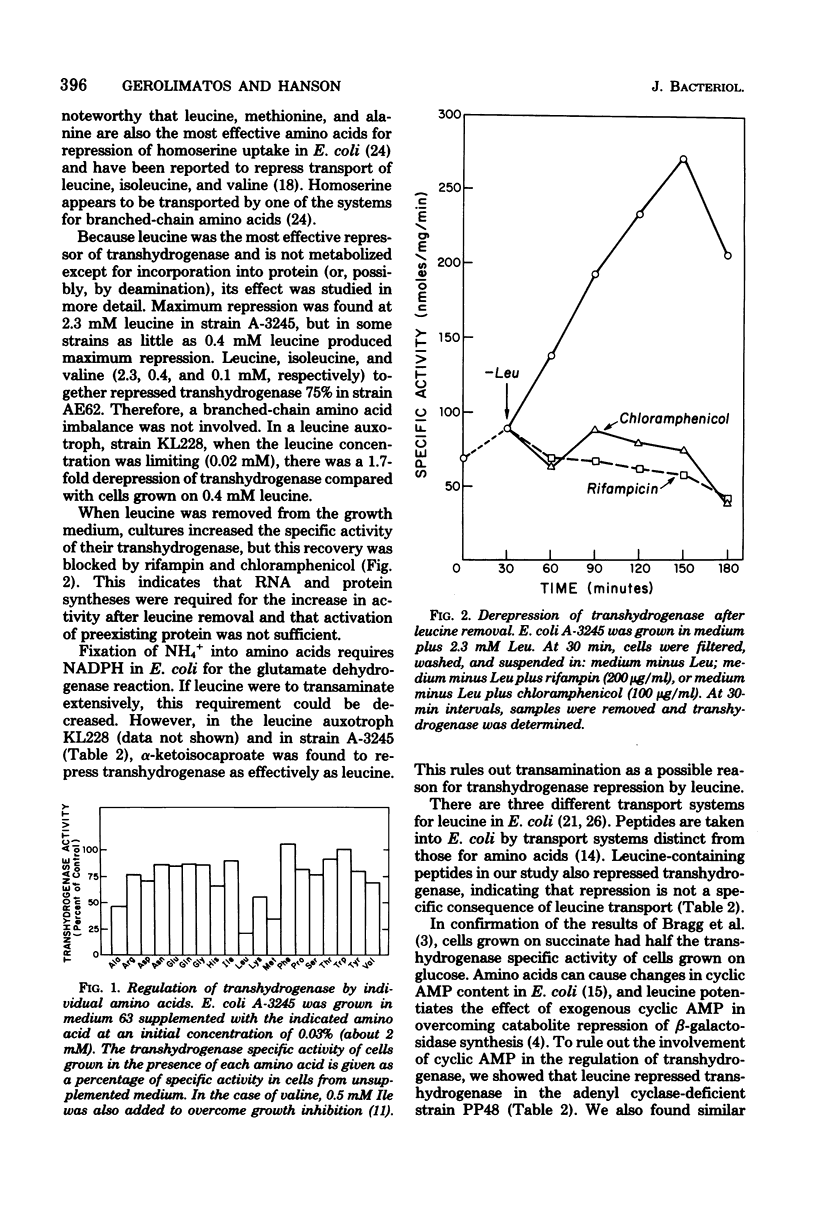
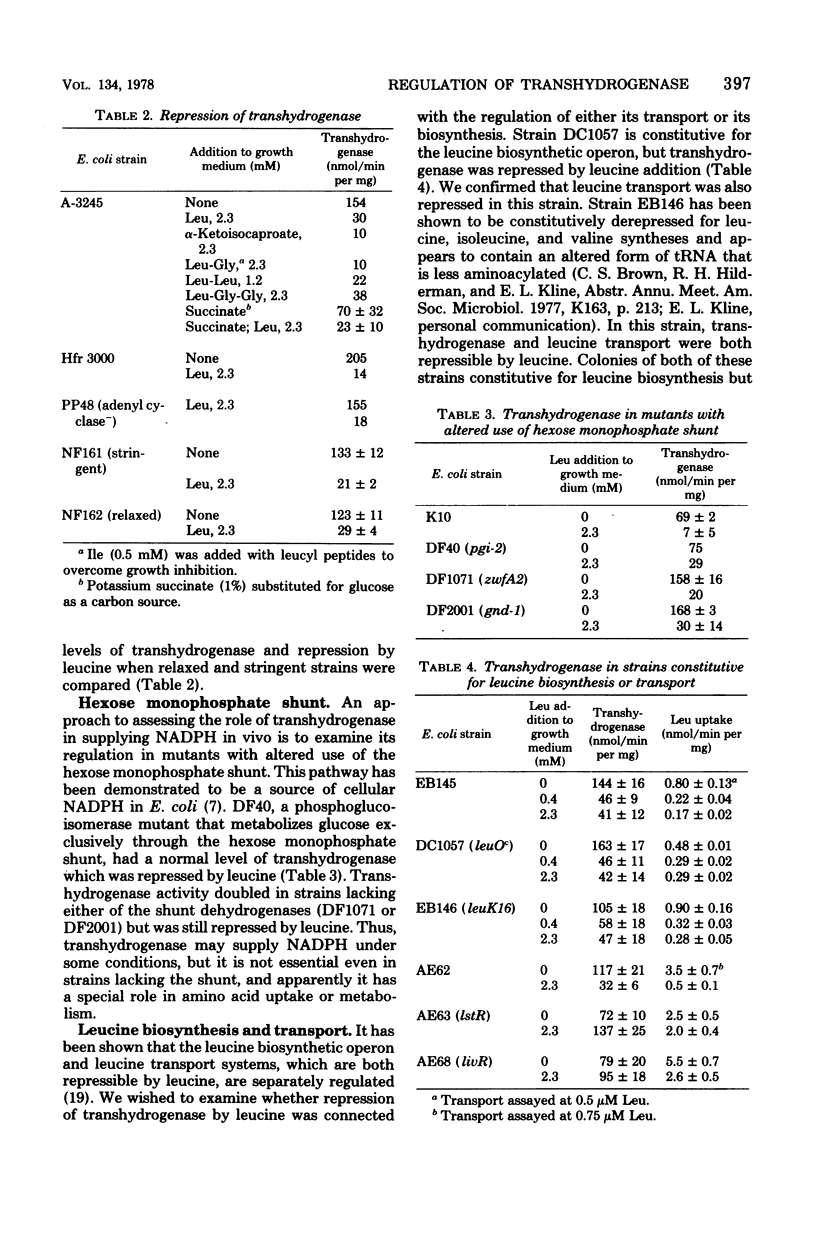
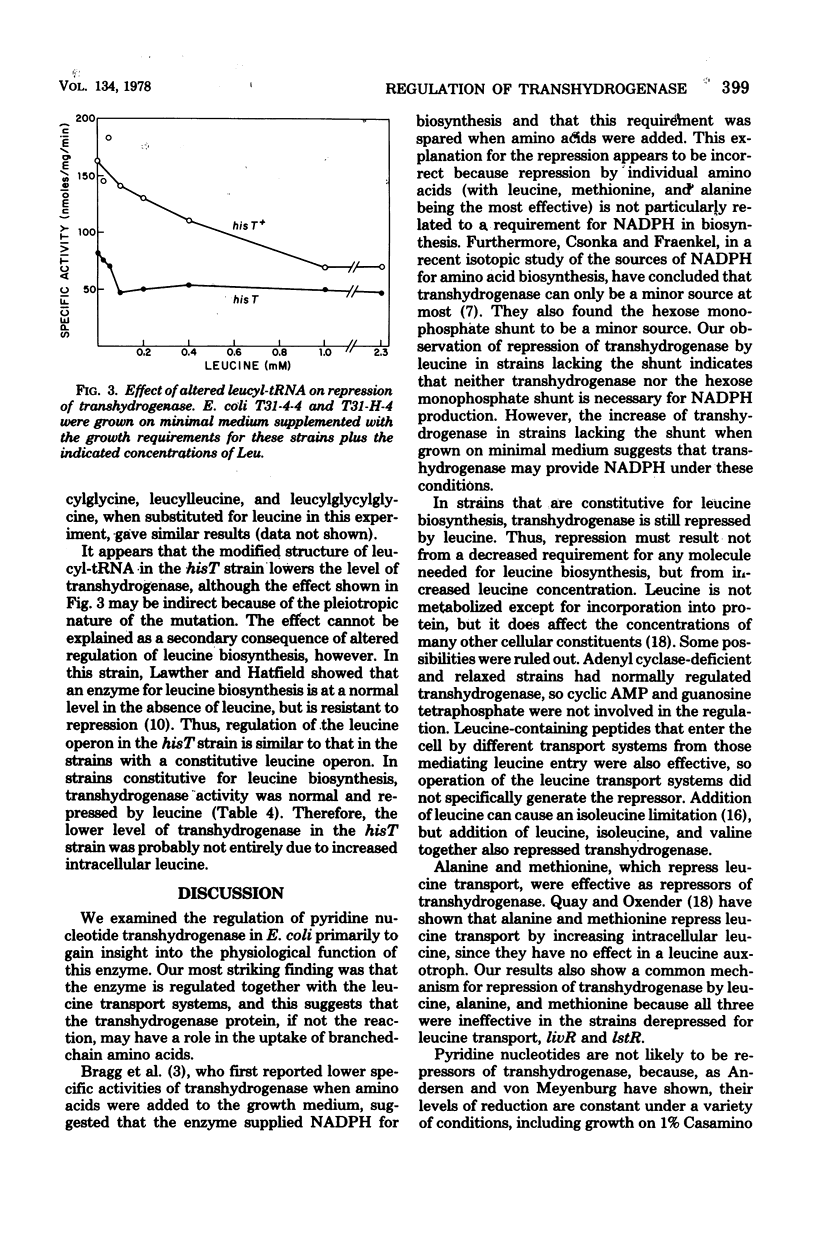
Addition of 0.1% casein hydrolysate to a minimal growth medium decreased membrane-bound transhydrogenase activity in Escherichia coli by about 80%. Of the amino acids added individually to the growth medium, only leucine and, to a lesser extent, methionine and alanine were effective, alpha-Ketoisocaproate- and leucine-containing peptides repressed the activity, and leucine also repressed activity in adenyl cyclase-deficient and relaxed strains. Derepression of transhydrogenase followed the removal of leucine from the growth medium and was sensitive to rifampin and chloramphenicol. A phosphoglucoisomerase-deficient strain that was forced to use the hexose monophosphate shunt exclusively had normal levels of transhydrogenase, which was repressed by leucine. Transhydrogenase activity doubled in mutants lacking either of the shunt dehydrogenases but was still repressed by leucine. In strains constitutive for the leucine biosynthetic operon, transhydrogenase was repressed by leucine but in strains livR and lst R, with leucine transport resistant to leucine repression, transhydrogenase was not repressed by leucine. These data suggest that transhydrogenase may have a function in the transport of branched-chain amino acids. In a hisT strain (which has altered leucyl-tRNA), transhydrogeanse was at a repressed level without the addition of leucine, suggesting that leucyl-tRNA may be involved in the regulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. B., von Meyenburg K. Charges of nicotinamide adenine nucleotides and adenylate energy charge as regulatory parameters of the metabolism in Escherichia coli. J Biol Chem. 1977 Jun 25;252(12):4151–4156. [PubMed] [Google Scholar]
- Anderson J. J., Quay S. C., Oxender D. L. Mapping of two loci affecting the regulation of branched-chain amino acid transport in Escherichia coli K-12. J Bacteriol. 1976 Apr;126(1):80–90. doi: 10.1128/jb.126.1.80-90.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg P. D., Davies P. L., Hou C. Function of energy-dependent transhydrogenase in Escherichia coli. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1248–1255. doi: 10.1016/0006-291x(72)90969-2. [DOI] [PubMed] [Google Scholar]
- Broman R. L., Goldenbaum P. E., Dobrogosz W. J. The effect of amino acids on the ability of cyclic AMP to reverse catabolite repression in Escherichia coli. Biochem Biophys Res Commun. 1970 May 11;39(3):401–406. doi: 10.1016/0006-291x(70)90591-7. [DOI] [PubMed] [Google Scholar]
- Bruni C. B., Colantuoni V., Sbordone L., Cortese R., Blasi F. Biochemical and regulatory properties of Escherichia coli K-12 hisT mutants. J Bacteriol. 1977 Apr;130(1):4–10. doi: 10.1128/jb.130.1.4-10.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Csonka L. N., Fraenkel D. G. Pathways of NADPH formation in Escherichia coli. J Biol Chem. 1977 May 25;252(10):3382–3391. [PubMed] [Google Scholar]
- Holmes W. M., Goldman E., Miner T. A., Hatfield G. W. Differential utilization of leucyl-tRNAs by Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1393–1397. doi: 10.1073/pnas.74.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline E. L. New amino acid regulatory locus having unusual properties in heterozygous merodiploids. J Bacteriol. 1972 Jun;110(3):1127–1134. doi: 10.1128/jb.110.3.1127-1134.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli strain K-12. J Bacteriol. 1962 Mar;83:624–630. doi: 10.1128/jb.83.3.624-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawther R. P., Hatfield W. Biochemical characterization of an Escherichia coli hisT strain. J Bacteriol. 1977 Apr;130(1):552–557. doi: 10.1128/jb.130.1.552-557.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L., Quay S. C. Regulation of leucine transport and binding proteins in Escherichia coli. J Cell Physiol. 1976 Dec;89(4):517–521. doi: 10.1002/jcp.1040890405. [DOI] [PubMed] [Google Scholar]
- Piovant M., Lazdunski C. Different cyclic adenosine 3',5'-monophosphate requirements for induction of beta-galactosidase and tryptophanase. Effect of osmotic pressure on intracellular cyclic adenosine 3,5-monophosphate concentrations. Biochemistry. 1975 May 6;14(9):1821–1825. doi: 10.1021/bi00680a003. [DOI] [PubMed] [Google Scholar]
- Quay S. C., Dick T. E., Oxender D. L. Role of transport systems in amino acid metabolism: leucine toxicity and the branched-chain amino acid transport systems. J Bacteriol. 1977 Mar;129(3):1257–1265. doi: 10.1128/jb.129.3.1257-1265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Kline E. L., Oxender D. L. Role of leucyl-tRNA synthetase in regulation of branched-chain amino-acid transport. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3921–3924. doi: 10.1073/pnas.72.10.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Oxender D. L. Regulation of branched-chain amino acid transport in Escherichia coli. J Bacteriol. 1976 Sep;127(3):1225–1238. doi: 10.1128/jb.127.3.1225-1238.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Oxender D. L., Tsuyumu S., Umbarger H. E. Separate regulation of transport and biosynthesis of leucine, isoleucine, and valine in bacteria. J Bacteriol. 1975 Jun;122(3):994–1000. doi: 10.1128/jb.122.3.994-1000.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADHAKRISHANAN A. N., WAGNER R. P., SNELL E. E. Biosynthesis of valine and i43soleucine, 3. alpha-Keto-beta-hydroxy acid reductase and alpha-hydroxy-beta-Keto acid reductoisomerase. J Biol Chem. 1960 Aug;235:2322–2331. [PubMed] [Google Scholar]
- Rahmanian M., Claus D. R., Oxender D. L. Multiplicity of leucine transport systems in Escherichia coli K-12. J Bacteriol. 1973 Dec;116(3):1258–1266. doi: 10.1128/jb.116.3.1258-1266.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson A. C., Freundlich M. Control of isoleucine, valine and leucine biosynthesis. 8. Mechanism of growth inhibition by leucine in relaxed and stringent strains of Escherichia coli K-12. Biochim Biophys Acta. 1970 Apr 14;208(1):87–98. doi: 10.1016/0304-4165(70)90051-6. [DOI] [PubMed] [Google Scholar]
- Templeton B. A., Savageau M. A. Transport of biosynthetic intermediates: regulation of homoserine and threonine uptake in Escherichia coli. J Bacteriol. 1974 Oct;120(1):114–120. doi: 10.1128/jb.120.1.114-120.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonder Haar R. A., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XIX. Inhibition of isoleucine biosynthesis by glycyl-leucine. J Bacteriol. 1972 Oct;112(1):142–147. doi: 10.1128/jb.112.1.142-147.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M. Leucine transport in Escherichia coli. The resolution of multiple transport systems and their coupling to metabolic energy. J Biol Chem. 1975 Jun 25;250(12):4477–4485. [PubMed] [Google Scholar]



