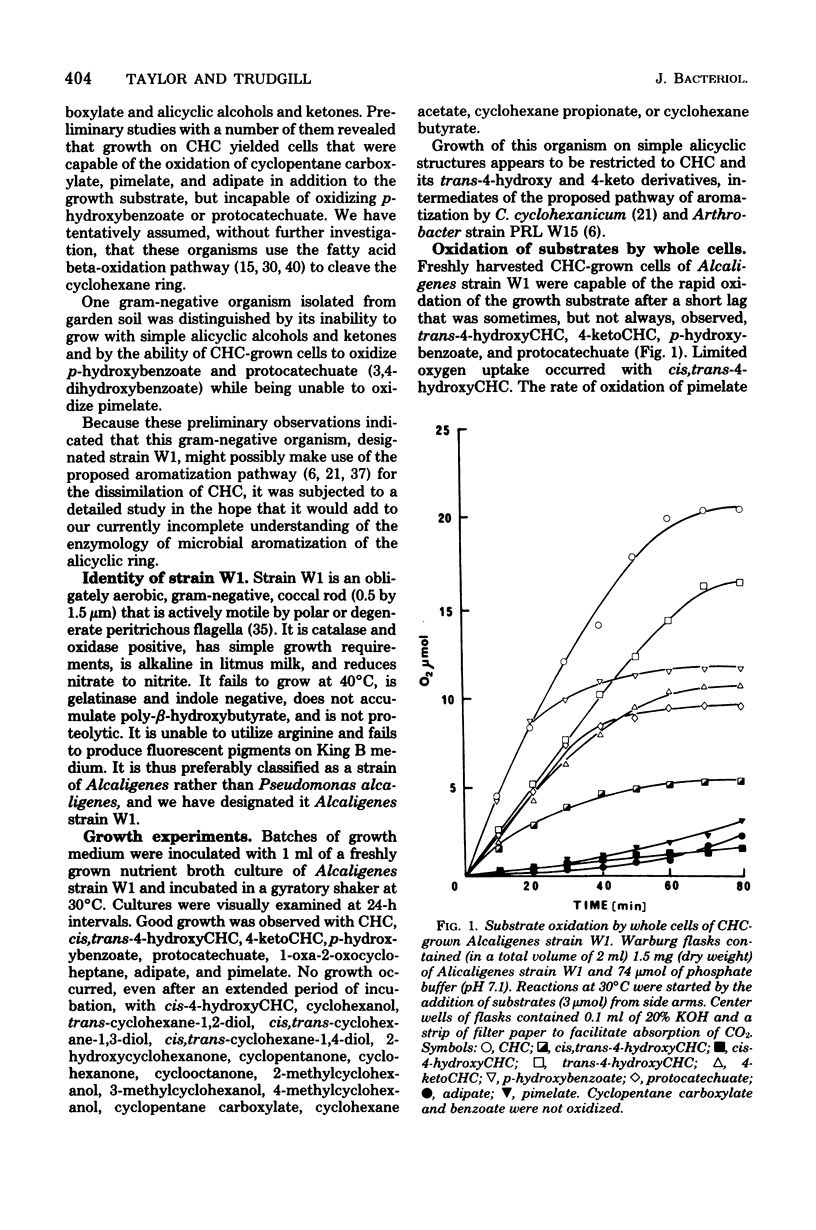
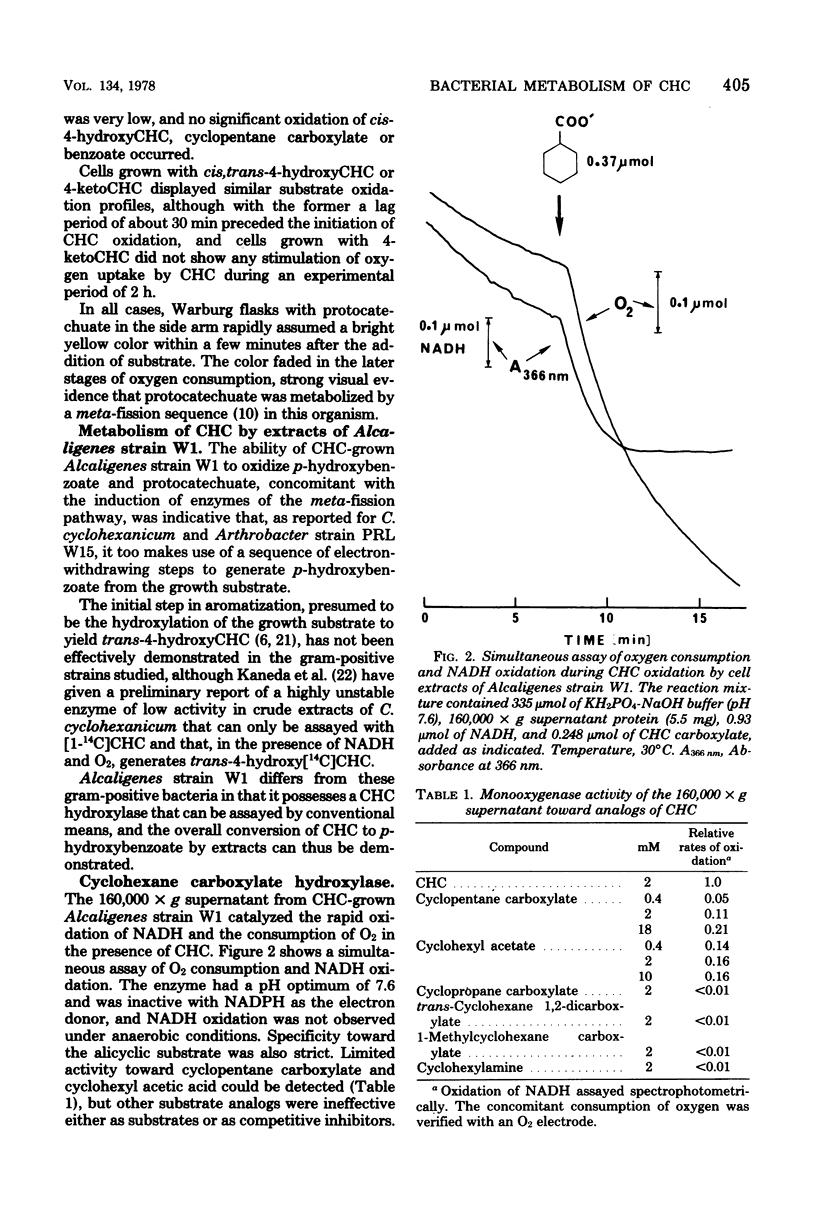
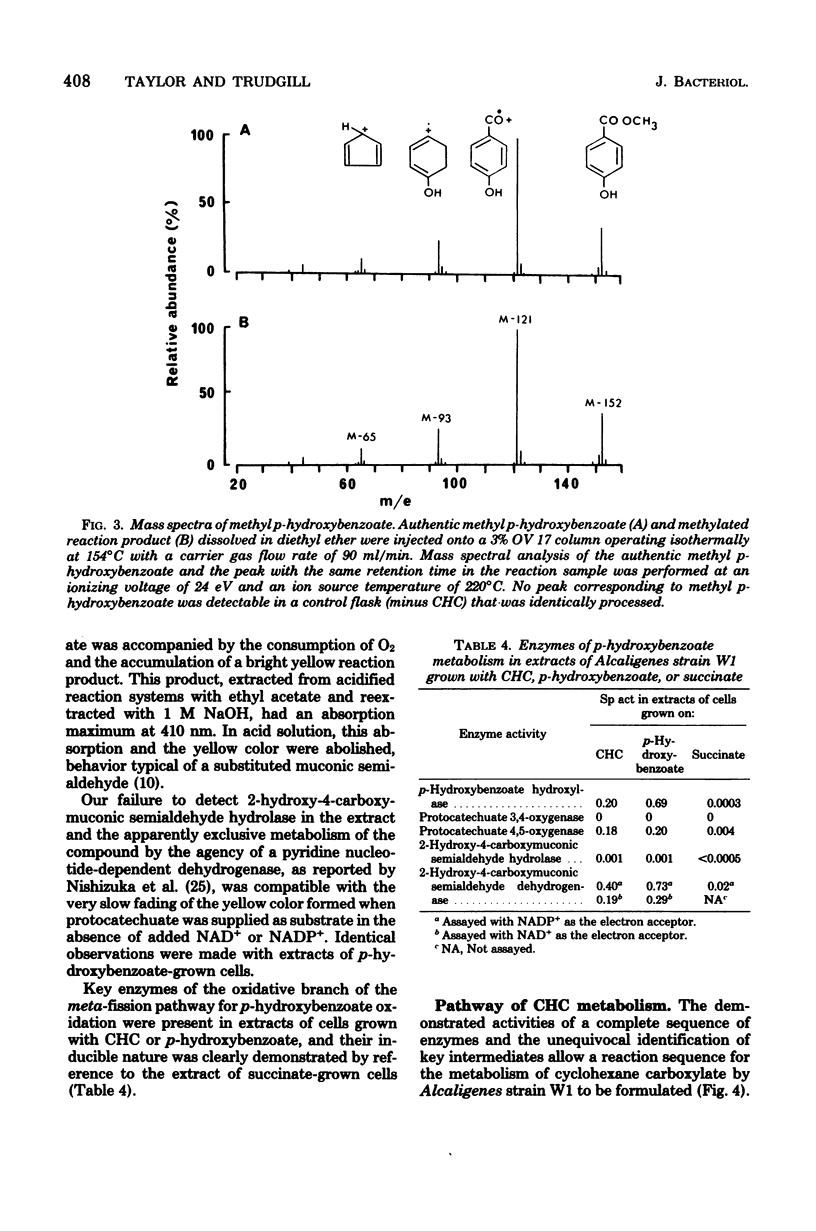
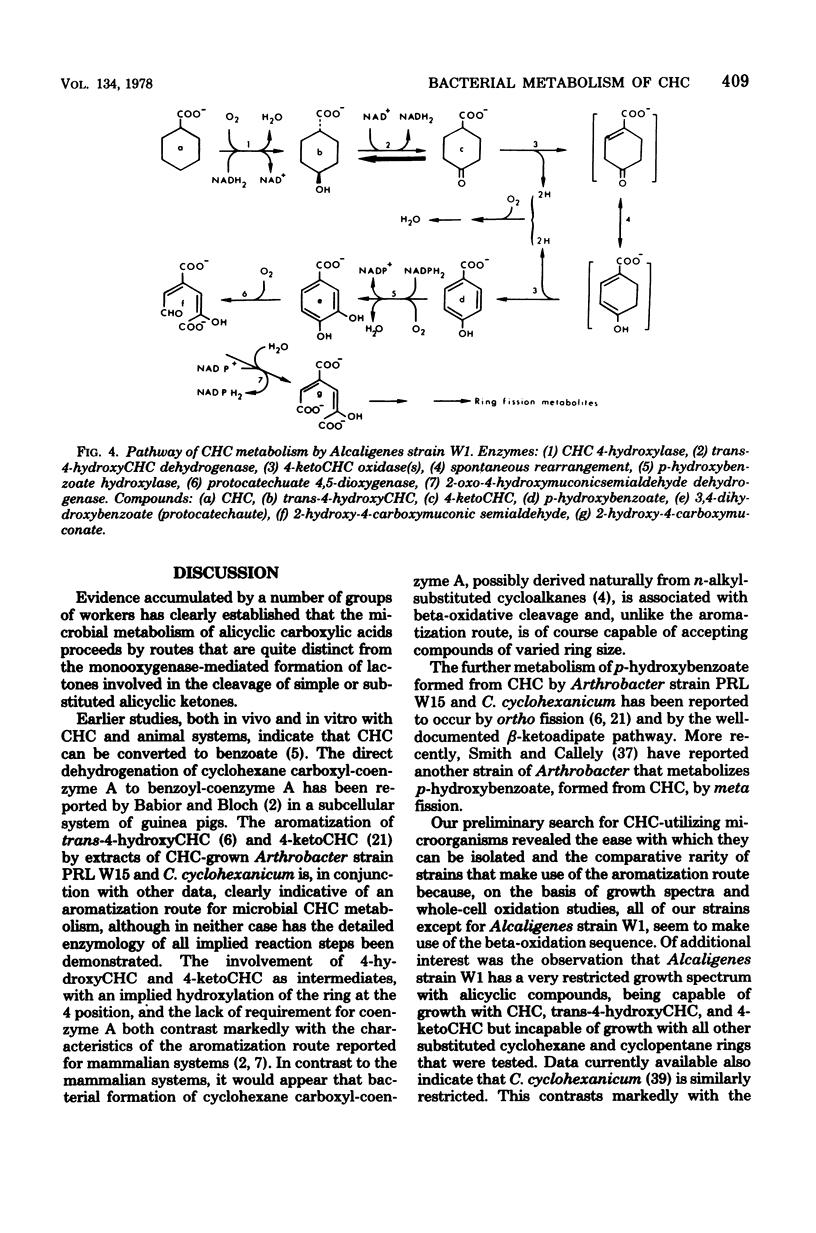
Abstract
Thirty-three microorganisms capable of growth with cyclohexane carboxylate as the sole source of carbon were isolated from mud, water, and soil samples from the Aberystwyth area. Preliminary screening and whole-cell oxidation studies suggested that, with one exception, all of the strains metabolized the growth substrate by beta-oxidation of the coenzyme A ester. This single distinctive strain, able to oxidize rapidly trans-4-hydroxycyclohexane carboxylate, 4-ketocyclohexane carboxylate, p-hydroxybenzoate, and protocatechuate when grown with cyclohexane carboxylate, was classified as a strain of Alcaligenes and given the number W1. Enzymes capable of converting cyclohexane carboxylate to p-hydroxybenzoate were induced by growth with the alicyclic acid and included the first unambiguous specimen of a cyclohexane carboxylate hydroxylase. Because it is a very fragile protein, attempts to stabilize the cyclohexane carboxylate hydroxylase so that a purification procedure could be developed have consistently failed. In limited studies with crude cell extracts, we found that hydroxylation occurred at the 4 position, probably yielding the trans isomer of 4-hydroxycyclohexane carboxylate. Simultaneous measurement of oxygen consumption and reduced nicotinamide adenine dinucleotide oxidation, coupled with an assessment of reactant stoichiometry, showed the enzyme to be a mixed-function oxygenase. Mass spectral analysis enabled the conversion of cyclohexane carboxylate to p-hydroxybenzoate by cell extracts to be established unequivocally, and all of our data were consistent with the pathway: cyclohexane carboxylate → trans-4-hydroxycyclohexane carboxylate → 4-ketocyclohexane carboxylate → p-hydroxybenzoate. The further metabolism of p-hydroxybenzoate proceeded by meta fission and by the oxidative branch of the 2-hydroxy-4-carboxymuconic semialde-hyde-cleaving pathway.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEER C. T., DICKENS F., PEARSON J. The aromatization of hydrogenated derivatives of benzoic acid in animal tissues. Biochem J. 1951 Feb;48(2):222–237. doi: 10.1042/bj0480222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Bloch K. Aromatization of cyclohexanecarboxylic acid. J Biol Chem. 1966 Aug 25;241(16):3643–3651. [PubMed] [Google Scholar]
- Beam H. W., Perry J. J. Microbial degradation and assimilation of n-alkyl-substituted cycloparaffins. J Bacteriol. 1974 May;118(2):394–399. doi: 10.1128/jb.118.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster D., Jones R. S., Parke D. V. The metabolism of cyclohexanecarboxylate in the rat. Biochem J. 1977 Jun 15;164(3):595–600. doi: 10.1042/bj1640595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., EVANS W. C., RIBBONS D. W. New pathways in the oxidative metabolism of aromatic compounds by microorganisms. Nature. 1960 Nov 12;188:560–566. doi: 10.1038/188560a0. [DOI] [PubMed] [Google Scholar]
- Dagley S., Geary P. J., Wood J. M. The metabolism of protocatechuate by Pseudomonas testosteroni. Biochem J. 1968 Oct;109(4):559–568. doi: 10.1042/bj1090559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J. F., Trudgill P. W. The metabolism of trans-cyclohexan-1,2-diol by an Acinetobacter species. Eur J Biochem. 1977 Mar 15;74(1):115–127. doi: 10.1111/j.1432-1033.1977.tb11373.x. [DOI] [PubMed] [Google Scholar]
- Donoghue N. A., Norris D. B., Trudgill P. W. The purification and properties of cyclohexanone oxygenase from Nocardia globerula CL1 and Acinetobacter NCIB 9871. Eur J Biochem. 1976 Mar 16;63(1):175–192. doi: 10.1111/j.1432-1033.1976.tb10220.x. [DOI] [PubMed] [Google Scholar]
- Donoghue N. A., Trudgill P. W. The metabolism of cyclohexanol by Acinetobacter NCIB 9871. Eur J Biochem. 1975 Dec 1;60(1):1–7. doi: 10.1111/j.1432-1033.1975.tb20968.x. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Evans W. C. The metabolism of aromatic compounds by Rhodopseudomonas palustris. A new, reductive, method of aromatic ring metabolism. Biochem J. 1969 Jul;113(3):525–536. doi: 10.1042/bj1130525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin M., Trudgill P. W. Purification and properties of cyclopentanone oxygenase of Pseudomonas NCIB 9872. Eur J Biochem. 1976 Mar 16;63(1):199–209. doi: 10.1111/j.1432-1033.1976.tb10222.x. [DOI] [PubMed] [Google Scholar]
- Griffin M., Trudgill P. W. The metabolism of cyclopentanol by Pseudomonas N.C.I.B. 9872. Biochem J. 1972 Sep;129(3):595–603. doi: 10.1042/bj1290595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbert C. C., Chung A. E. Metabolism of cyclopropanecarboxylic acid. A new role for carnitine. J Biol Chem. 1974 Feb 25;249(4):1026–1030. [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Kaneda T. Enzymatic aromatization of 4-ketocyclohexanecarboxylic acid to p-hydroxybenzoic acid. Biochem Biophys Res Commun. 1974 May 7;58(1):140–144. doi: 10.1016/0006-291x(74)90902-4. [DOI] [PubMed] [Google Scholar]
- Norris D. B., Trudgill P. W. The metabolism of cyclohexanol by Nocardia globerula CL1. Biochem J. 1971 Feb;121(3):363–370. doi: 10.1042/bj1210363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- PRAIRIE R. L., TALALAY P. Enzymatic formation of testololactone. Biochemistry. 1963 Jan-Feb;2:203–208. doi: 10.1021/bi00901a039. [DOI] [PubMed] [Google Scholar]
- RHODES M. E. The cytology of Pseudomonas spp. as revealed by a silver-plating staining method. J Gen Microbiol. 1958 Jun;18(3):639–648. doi: 10.1099/00221287-18-3-639. [DOI] [PubMed] [Google Scholar]
- ROSENBERGER R. F., ELSDEN S. R. The yields of Streptococcus faecalis grown in continuous culture. J Gen Microbiol. 1960 Jun;22:726–739. doi: 10.1099/00221287-22-3-726. [DOI] [PubMed] [Google Scholar]
- Rho E. M., Evans W. C. The aerobic metabolism of cyclohexanecarboxylic acid by Acinetobacter anitratum. Biochem J. 1975 Apr;148(1):11–15. doi: 10.1042/bj1480011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANIER R. Y., INGRAHAM J. L. Protocatechuic acid oxidase. J Biol Chem. 1954 Oct;210(2):799–808. [PubMed] [Google Scholar]
- Sala-Trepat J. M., Murray K., Williams P. A. The metabolic divergence in the meta cleavage of catechols by Pseudomonas putida NCIB 10015. Physiological significance and evolutionary implications. Eur J Biochem. 1972 Jul 24;28(3):347–356. doi: 10.1111/j.1432-1033.1972.tb01920.x. [DOI] [PubMed] [Google Scholar]
- Schiller J. G., Chung A. E. The metabolism of cyclopropanecarboxylic acid. J Biol Chem. 1970 Nov 10;245(21):5857–5864. [PubMed] [Google Scholar]
- Smith D. I., Callely A. G. The microbial degradation of cyclohexane carboxylic acid. J Gen Microbiol. 1975 Nov;91(1):210–212. doi: 10.1099/00221287-91-1-210. [DOI] [PubMed] [Google Scholar]
- Tokuyama T., Kaneda T. Corynebacterium cyclohexanicum n. sp.: a cyclohexanecarboxylic acid utilizing bacterium. Can J Microbiol. 1973 Aug;19(8):937–942. doi: 10.1139/m73-150. [DOI] [PubMed] [Google Scholar]
- Whittle P. J., Lunt D. O., Evans W. C. Anaerobic photometabolism of aromatic compounds by Rhodopseudomonas sp. Biochem Soc Trans. 1976;4(3):490–491. doi: 10.1042/bst0040490. [DOI] [PubMed] [Google Scholar]
- Williams R. J., Evans W. C. The metabolism of benzoate by Moraxella species through anaerobic nitrate respiration. Evidence for a reductive pathway. Biochem J. 1975 Apr;148(1):1–10. doi: 10.1042/bj1480001a. [DOI] [PMC free article] [PubMed] [Google Scholar]


