Abstract
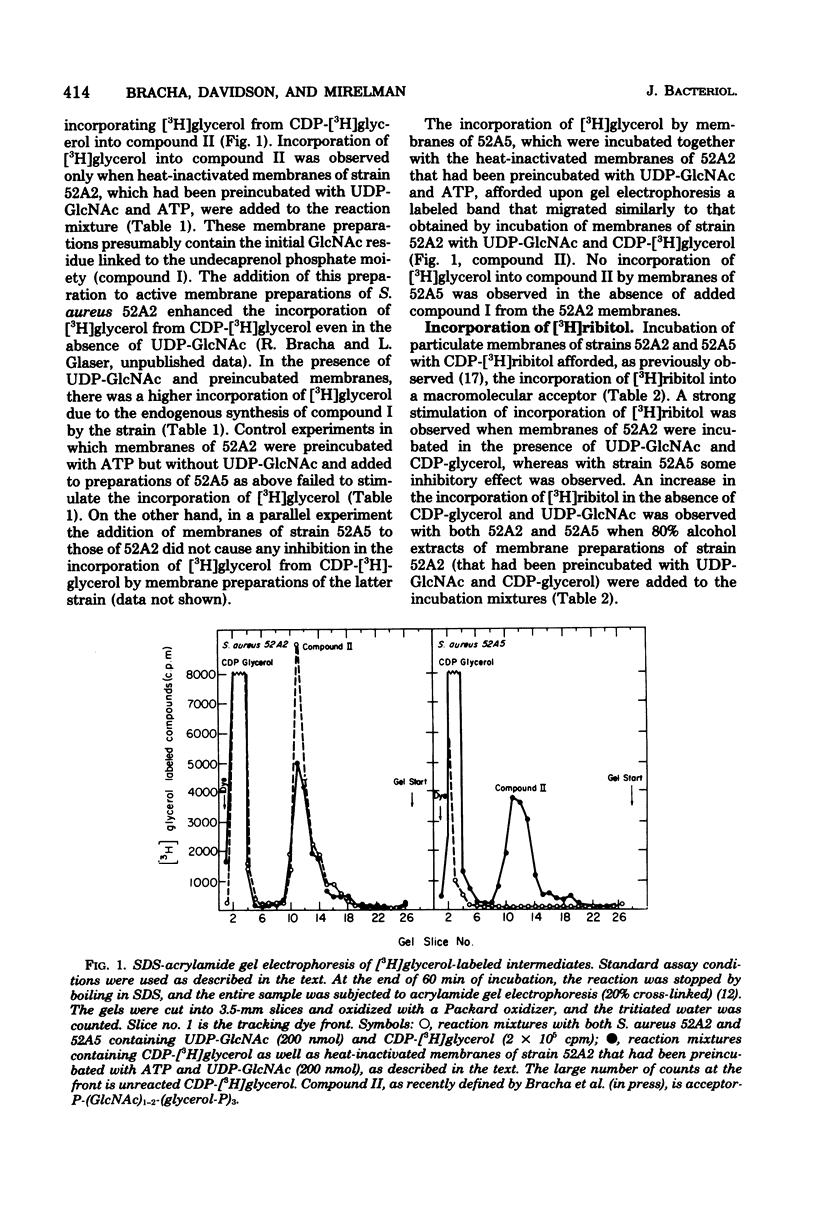
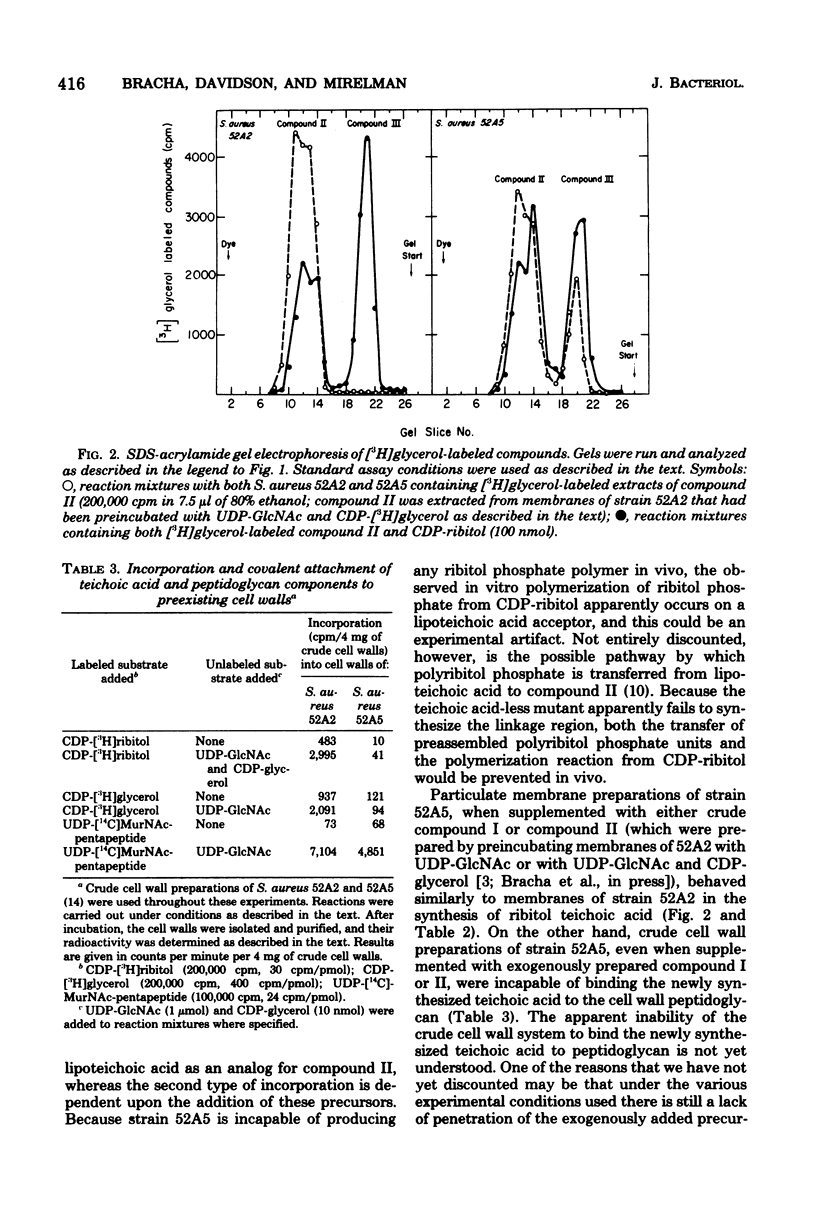
The biosynthesis of the linkage region between peptidoglycan and the ribitol teichoic acid was investigated in the bacteriophage-resistant, teichoic acid-less mutant Staphylococcus aureus 52A5 (Chatterjee et al., J. Bacteriol. 100:846--853, 1969). Membrane preparations of this strain were found to be incapable of forming the first intermediate of the biosynthetic pathway, namely, the transfer of N-acetyl-D-glucosamine (GlcNAc) from UDP-GlcNAc to the acceptor molecule, which presumbably is undecaprenol phosphate (R. Bracha and L. Glaser, Biochem. Biophys. Res. Commun. 72:1091--1098, 1976). The addition of heat-inactivated membrane preparations of S. aureus 52A2 (which normally has ribitol teichoic acid) that had been preincubated with UDP-GlcNAc to membranes of strain 52A5 enabled the synthesis of teichoic acid. These data suggest that the mutational defect in the teichoic acid-less organism is in the synthesis of the first compound of the linkage unit, and this is apparently the reason for its absence in the cell walls.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baddiley J. Teichoic acids in cell walls and membranes of bacteria. Essays Biochem. 1972;8:35–77. [PubMed] [Google Scholar]
- Bettinger G. E., Chatterjee A. N., Young F. E. Incorporation of N-acetyl-D-glucosamine from UDP-N-acetyl-D-glucosamine by isolated membranes of Bacillus subtilis. Identification of undecaprenyl poly(N-acetylglucosaminyl pyrophosphate). J Biol Chem. 1977 Jun 25;252(12):4118–4124. [PubMed] [Google Scholar]
- Bracha R., Glaser L. An intermediate in telchoic acid biosynthesis. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1091–1098. doi: 10.1016/s0006-291x(76)80244-6. [DOI] [PubMed] [Google Scholar]
- Bracha R., Glaser L. In vitro system for the synthesis of teichoic acid linked to peptidoglycan. J Bacteriol. 1976 Mar;125(3):872–879. doi: 10.1128/jb.125.3.872-879.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N., Mirelman D., Singer H. J., Park J. T. Properties of a novel pleiotropic bacteriophage-resistant mutant of Staphylococcus aureus H. J Bacteriol. 1969 Nov;100(2):846–853. doi: 10.1128/jb.100.2.846-853.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N. Use of bacteriophage-resistant mutants to study the nature of the bacteriophage receptor site of Staphylococcus aureus. J Bacteriol. 1969 May;98(2):519–527. doi: 10.1128/jb.98.2.519-527.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley J., Archibald A. R., Baddiley J. A linkage unit joining peptidoglycan to teichoic acid in Staphylococcus aureus H. FEBS Lett. 1976 Jan 15;61(2):240–242. doi: 10.1016/0014-5793(76)81047-2. [DOI] [PubMed] [Google Scholar]
- Coley J., Archibald R., Baddiley J. The presence of N-acetylglucosamine 1-phosphate in the linkage unit that connects teichoic acid to peptidoglycan in Staphylococcus aureus. FEBS Lett. 1977 Aug 15;80(2):405–407. doi: 10.1016/0014-5793(77)80486-9. [DOI] [PubMed] [Google Scholar]
- Fielder F., Glaser L. Assembly of bacterial cell walls. Biochim Biophys Acta. 1973 Dec 28;300(4):467–485. doi: 10.1016/0304-4157(73)90016-6. [DOI] [PubMed] [Google Scholar]
- Hancock I. C., Wiseman G., Baddiley J. Biosynthesis of the unit that links teichoic acid to the bacterial wall: inhibition by tunicamycin. FEBS Lett. 1976 Oct 15;69(1):75–80. doi: 10.1016/0014-5793(76)80657-6. [DOI] [PubMed] [Google Scholar]
- Heckels J. E., Archibald A. R., Baddiley J. Studies on the linkage between teichoic acid and peptidoglycan in a bacteriophage-resistant mutant of Staphylococcus aureus H. Biochem J. 1975 Sep;149(3):637–647. doi: 10.1042/bj1490637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mirelman D. A rapid and simple procedure for the preparation of the two bacterial cell wall peptidoglycan nucleotide precursors labeled in their amino sugars. Anal Biochem. 1976 Feb;70(2):424–429. doi: 10.1016/0003-2697(76)90465-6. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Sharon N. Biosynthesis of peptidoglycan by a cell wall preparation of Staphylococcus aureus and its inhibition by penicillin. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1909–1917. doi: 10.1016/0006-291x(72)90069-1. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Shaw D. R., Park J. T. Nature and origins of phosphorus compounds in isolated cell walls of Staphylococcus aureus. J Bacteriol. 1971 Jul;107(1):239–244. doi: 10.1128/jb.107.1.239-244.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. R., Mirelman D., Chatterjee A. N., Park J. T. Ribitol teichoic acid synthesis in bacteriophage-resistant mutants of Staphylococcus aureus H. J Biol Chem. 1970 Oct 10;245(19):5101–5106. [PubMed] [Google Scholar]
- Wyke A. W., Ward J. B. Biosynthesis of wall polymers in Bacillus subtilis. J Bacteriol. 1977 Jun;130(3):1055–1063. doi: 10.1128/jb.130.3.1055-1063.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke A. W., Ward J. B. The biosynthesis of muramic acid phosphate in Bacillus licheniformis. FEBS Lett. 1977 Feb 1;73(2):159–163. doi: 10.1016/0014-5793(77)80971-x. [DOI] [PubMed] [Google Scholar]



