Abstract
An isoluminant chromatic display is a color display in which the component colors have been so carefully equated in luminance that they stimulate only color-sensitive perceptual mechanisms and not luminance-sensitive mechanisms. The nature of the mechanism by which isoluminant chromatic motion is perceived is an important issue because color and motion processing historically have been associated with different neural pathways. Here we show that isoluminant chromatic motion (i) fails a pedestal test, (ii) has a temporal tuning function that declines to half-amplitude at 3–6 Hz, and (iii) is perceived equally well when the entire motion sequence is presented monocularly (entire motion sequence to one eye) versus interocularly (the frames of motion sequence alternate between eyes so that neither eye individually could perceive motion). These three characteristics indicate that chromatic motion is detected by the third-order motion system. Based on this theory, it was possible to take a moving isoluminant red–green grating and, by simply increasing the chromatic contrast of the green component, to generate the full gamut of motion percepts, from compelling smooth motion to motion standstill. The perception of motion standstill when the third-order mechanism is nullified indicates that there is no other motion computation available for purely chromatic motion. It follows that isoluminant chromatic motion is not computed by specialized chromatic motion mechanisms within a color pathway but by the third-order motion system at a brain level where binocular inputs of form, color, depth, and texture are simultaneously available and where selective attention can exert a major influence.
An isoluminant chromatic display is a color display in which the component colors have been so carefully equated in luminance that they stimulate only color-sensitive perceptual mechanisms and not luminance-sensitive mechanisms. When an isoluminant display—e.g., a grating of alternating red and green stripes—is moved, the apparent movement is often reported to be perceptually different from the movement of ordinary displays, being neither as smooth nor as quick (1–8). In some instances, “motion standstill” of isoluminant displays has been reported (1–5), a phenomenon in which the moving display appears motionless but nevertheless is perceived as occupying different positions from time to time. Here we use a pedestal paradigm, the temporal tuning function, and a comparison between observers’ performance in monocular and interocular presentations to infer that chromatic motion is perceived by the third-order motion system. The apparently poor quality of isoluminant motion is not intrinsic to chromatic motion but merely to the chromatic stimuli that have heretofore been generated. By manipulating the ratio of red-to-green contrast in accordance with the theory of third-order motion, we produce high-contrast, easily visible, isoluminant displays that exhibit the full gamut of motion percepts: from normal, easily perceived motion to motion-standstill.
The nature of the mechanism or mechanisms by which isoluminant motion is perceived is an important issue because color and motion processing historically have been associated with different neural pathways: color with parvo or P-pathways, motion with magno or M-pathways. There is compelling evidence from animal research that color and motion are processed in separate neural pathways (9–12), implying either (i) that no percept of motion can arise from movement of purely chromatic modulations or (ii) that there is a separate neural motion computation within the color pathways. On the other hand, although it has occasionally been stated that there is no isoluminant motion perception (ref. 13, p. 161), there are obvious demonstrations from human psychophysics of the perceived motion of isoluminant stimuli (14–16). Although the various degradations of motion perceived in isoluminant stimuli have led to speculations about special mechanisms for chromatic motion (17–21), lack of convincing experimental paradigms and results has left completely unresolved the question of how and where isoluminant motion might be computed, and there are supporters for all theoretical stands (22–29).
We propose to resolve the question of the mechanism of isoluminant chromatic motion within the architecture of first-, second-, and third-order motion processing (30–32). To review: First-order motion is computed from the movement of areas defined by their luminance; second-order motion is computed from movement of areas defined by luminance variance (texture-contrast); and third-order motion is computed from movement of areas defined as figure relative to the background. Third-order motion (unlike first- or second-order motion, which are computed primarily monocularly) is independent of the eye of the origin of successive moving frames, is highly vulnerable to attentional manipulations, and is sensitive to an incredibly wide range of stimuli (33–35). We will show here that isoluminant motion is computed by the third-order motion system, and only by the third-order motion system.
METHODS
Stimuli and Isoluminant Calibration
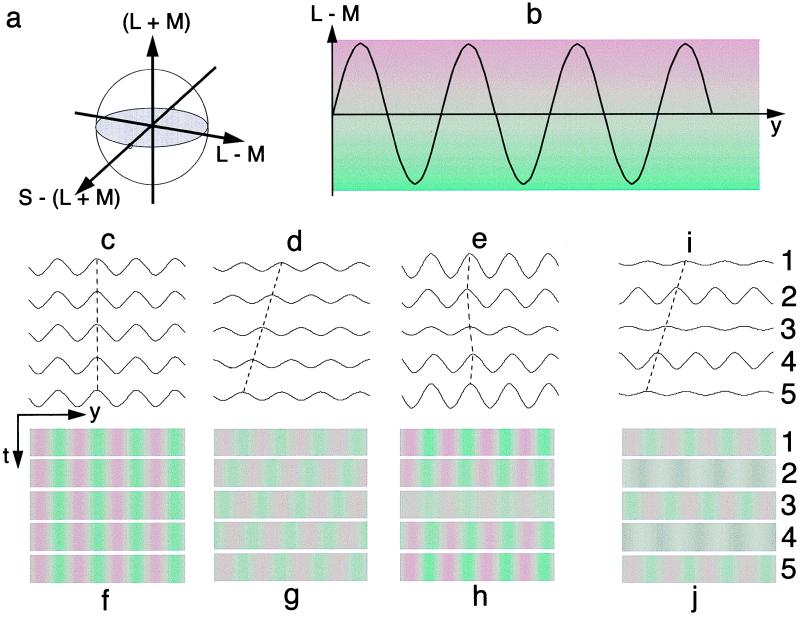
Stimuli.§ Red–green sinewave gratings (Fig.
1c) were generated by modulating along the L − M (Long-Medium wavelength) cardinal axis in color space (38, 39). To minimize chromatic aberration (40–42), gratings were horizontal. The background was white at 17.4 cd/m2 at a chromaticity (x, y) = (0.294, 0.314) for a CIE standard observer. At modulation depth of 8%, the reddest point on the grating has chromaticity (0.341, 0.304), the greenest, (0.242, 0.352). The moving sinewave grating consists of five isoluminant frames with a consistent phase shift of 90 degrees between successive frames. At the viewing distance of 60 cm, the grating has a spatial frequency of 0.5 c/degree and occupies 5.7 × 5.7 degrees. To produce motion stimuli at different temporal frequencies, the frames are shown with different numbers of screen refreshes. Four different temporal frequencies were used: 0.94, 1.88, 3.75, and 7.5 Hz. In each trial, the motion stimulus contains only five frames.
Improved Calibration.
It is critical that all of the “isoluminant” stimuli be truly isoluminant with respect to human motion perception. The calibration proceeded in two phases: The first phase was a static calibration of the display for isoluminance by standard methods (37); the second, critical phase was a dynamic calibration of each particular stimulus by means of the improved calibration procedure (43) described below.
After standard isoluminance calibration of the display system, a method of constant stimuli was used to estimate modulation amplitudes that yielded 90% correct motion–direction thresholds for the red–green gratings at each of the four temporal frequencies in the experiment. These 90% correct motion stimuli then were calibrated dynamically (Fig. 1 i and j). The calibration display consisted of five frames in which the odd frames are candidate isoluminant red–green sinewave gratings; the even frames are luminance (white/black) sinewave modulations; and the phase shift between successive frames is 90 degrees (Fig. 1 i and j). No consistent motion would be perceived in such a display if the isoluminant frames were truly isoluminant. However, if there were residual luminance contamination in the isoluminant frames, consistent motion would be perceived between the luminance contamination component in the odd frames and the luminance sinewaves in the even frames. As a practical matter, motion of the 90% correct gratings was nearly always perceived in the calibration display, indicating the ubiquitous presence of residual luminance contamination.
Figure 1.
Experimental Setup. (a) Color space and its three cardinal axis: luminance (L + M), red–green (L − M), and blue–yellow [S − (L + M)]. L, long (red); M, middle (green); S, short (blue) wavelength-sensitive cones. (b) Isoluminant spatial sinusoidal modulation along the L − M color axis. (c and f) The pedestal: five frames of an isoluminant stationary sinewave grating. (d and g) The moving sine grating: five isoluminant frames with a phase shift of 90 degrees between successive frames. (e and h) Pedestaled stimulus: summation of c and f and d and g. In pedestaled motion, features such as peaks, valleys, and zero crossings simply wobble back and forth periodically over time. Only wobble, not the constant linear motion of the motion stimulus, could be perceived if motion were computed by an algorithm that first extracted the features. On the other hand, Lu and Sperling (30) proved that, if the perceptual mechanism used a motion–energy computation, human observers would perceive motion equally from the pedestaled motion stimulus and the non-pedestaled motion stimulus. (i and j) Calibration stimulus: a five-frame display in which the odd frames are isoluminant sinewave gratings; the even frames are luminance sinewave modulations; and the phase shift between successive frames is 90 degrees. No consistent motion would be perceived in such a display if the isoluminant frames were truly isoluminant. In the actual experiments, the gratings were horizontal to avoid pixel-to-pixel intensity dependencies in the display monitor and to minimize chromatic aberration (40–42).
To each “candidate” 90%-correct motion stimulus in the calibration display, a small amount of luminance modulation was added to cancel apparent motion. This is the procedure of Cavanagh and Anstis (44). The canceling modulation was chosen from one of two luminance phases and five luminance amplitudes. Forty motion trials were conducted at each phase and amplitude, and this entire procedure was repeated for each temporal frequency (a total of 1,600 trials). The data were used to determine the added luminance needed to precisely cancel the residual luminance in the candidate stimuli. Although the display was already nominally isoluminant (according to the static calibration), there was always residual luminance contamination to be cancelled. Luminance resolution of one part in a thousand is required to achieve isoluminance. Each dynamically calibrated 90% stimulus then was used to construct a new set of isoluminant stimuli with smaller (and occasionally larger) modulation amplitudes.
The improvement to the Cavanagh-Anstis procedure (44) arises from a motion–energy analysis (43). The motion energy in a particular direction in the calibration display is proportional to the product of the modulation amplitudes of the even and odd frames (45, 46). By choosing the amplitude of the even, luminance-modulated frames to be >10× greater than the threshold amplitude for luminance motion perception, a luminance contamination of 1/10 threshold in the nominally isoluminant display would produce consistent apparent motion in the calibration display. Thus, reducing apparent motion in the calibration display to threshold guarantees a luminance contamination of <1/10 threshold in the isoluminant chromatic display. That is, even 10× more luminance contamination would still be below threshold. A second, critical aspect of the calibration is that it is repeated when any aspect of the display is changed—when the temporal frequency is changed or when a pedestal is added (see Discussion). Every calibration (each temporal frequency, modulation amplitude, pedestal and no pedestal) is repeated for each observer.
Plan.
To our knowledge, isoluminant chromatic stimuli of this certifiable purity have not been studied previously. Therefore, it is of interest to note that color-normal observers easily perceive motion when these isoluminant stimuli are moved. Three experiments are designed to determine how isoluminant chromatic motion is perceived in terms of first-, second-, and third-order motion processes. Experiment 1 investigates the temporal tuning function for isoluminant chromatic stimuli and finds that it precisely matches the previously measured tuning function for third-order motion (30). Experiment 2 determines whether the stimuli can pass the pedestal test (explained below). They fail the pedestal test, which is diagnostic of third-order motion. Experiment 3 investigates whether motion is perceived differently when stimuli are presented monocularly (all of the frames to one eye) or interocularly (frames alternate between eyes). First- and second-order motion perception fails in such interocular displays; third-order motion is equally good in monocular and interocular displays (30). After the formal experiments, a demonstration of motion standstill, a perceptual illusion in which objectively moving stimuli appear to standstill, is described. The theory of third-order motion processing predicts that an adjustment can be made of these isoluminant chromatic stimuli that will produce motion standstill for every observer. And an opposite adjustment would produce clear, easily perceived motion.
THREE EXPERIMENTS
Observers, Task.
Three naive observers and the second author participated in the experiments. All of the observers had corrected-to-normal vision. Before each experimental session, the observer adapted to the background for ≈3 minutes. The task was a forced-choice judgment of upward vs. downward motion direction. Auditory feedback was given after each correct response. In all experiments, trials for the four temporal frequencies were intermixed.
Experiment 1: Temporal Tuning Functions
Procedure.
To measure the temporal tuning function, the twice-calibrated stimuli were used with the method of constant stimuli (40 trials at each of five contrast levels for each of the four temporal frequencies, a total of 800 trials) to determine a 75% threshold amplitude for motion–direction discrimination. Each of these new 75% threshold stimuli was tested (its third calibration) to ensure that there still was no measurable luminance contamination, and there was none.
Results.
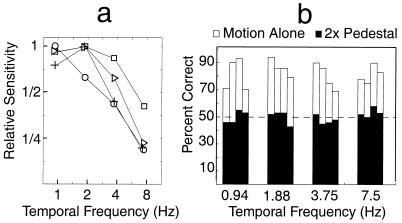
Fig. 2 shows the measured relative sensitivity against temporal frequency, i.e., the temporal tuning functions, for four observers. All of the temporal tuning functions are of “lowpass” shape. The frequency at which relative sensitivity is reduced by 1/2 falls between 3 and 6 Hz, which is consistent with the literature on color motion (47) and is also an excellent match to previously measured third-order motion tuning functions (30).
Figure 2.
(a) Temporal tuning functions: relative sensitivity (minimum 75% threshold amplitude divided by 75% threshold amplitude) as a function of temporal frequency for each of four observers. All of the functions are of lowpass character, with relative sensitivity falling to 1/2 of maximum sensitivity at 3–6 Hz. These data perfectly match previous measured temporal tuning functions of the third-order system. (b) Results of isoluminant pedestal tests for the same four observers: pedestaled vs. motion stimuli alone. Although observers could make reliable, correct motion–direction judgments in the absence of pedestal (69–94% correct depending on the actual amplitude of the chromatic modulation), adding a 2× pedestal reduced to chance the observers’ ability to perceive consistent motion from isoluminant displays.
Experiment 2: Pedestal Test
The pedestal test involves two stimuli: a moving sinewave grating (Fig. 1 d and g) and a stationary replica of the same grating (the pedestal; Fig. 1 c and f). When the stationary pedestal has a larger amplitude than the moving grating (typically 2×), the sum of the two stimuli is a sinewave in which the peaks wobble back and forth but do not move in a consistent direction (Fig. 1 e and h). Insofar as a motion detection algorithm depends on features such as peaks, valleys, and zero crossings to compute motion, it would fail with the pedestaled motion inputs. On the other hand, motion–energy algorithms are immune to pedestals under the conditions of the present experiments (30, 32). A motion–energy algorithm can extract motion equally well from pedestaled and nonpedestaled stimuli. In fact, human observers are immune to pedestals (no difference in motion–direction thresholds between pedestaled and unpedestaled stimuli) in luminance (first-order) and texture-contrast (second-order) motion, which is consistent with a motion–energy theory of first- and second-order motion (30, 45, 46).
On the other hand, humans perceive motion in a variety of exotic stimuli that fail the pedestal test: interocular stimuli, dynamic random dot stereomotion, and motion-from-motion (30, 48–51). The motion perception mechanism that detects these kinds of motion has been termed “third-order motion” perception, and it differs from first- and second-order motion perception in the preprocessing of the stimulus before the motion computation (see below) and in being slower (lower temporal half-amplitude frequency) (30).
Procedure.
We implement the pedestaled motion paradigm as follows: (i) determine each observer’s threshold amplitude for the discrimination of an upward from a downward moving sinewave grating; and (ii) add a pedestal with twice this measured threshold amplitude and re-determine the percent of correct motion–direction judgments. If the judgment were based on the output of a motion–energy computation, we would expect the observer’s accuracy of motion–direction judgments to be identical with and without the pedestal. On the other hand, if the motion–direction computation were based on stimulus features (peaks, valleys, zero-crossings, etc), the pedestaled motion would appear oscillatory, and it would be impossible for observers to judge motion direction of the test.
Three conditions were tested: motion stimulus at ≈75% threshold (with luminance correction), pedestaled motion stimulus in which the amplitude of the pedestal was twice that of the motion stimulus at threshold, and a calibration display (Fig. 1 i and j) with its odd frames made of the same motion stimulus frames at 75% threshold (with luminance correction). There were 80 trials per condition times each of four temporal frequencies (960 trials). All trials were intermixed. The average modulation depth for the isoluminant motion stimulus was 0.7, 0.7, 1.0, and 2.4% for the four temporal frequencies. Observers’ performance in the third condition (calibration verification) was always statistically at chance.
Results.
The results of the pedestal experiment are absolutely clear. Observers make reliably correct motion–direction judgments in the absence of pedestal (69–94% correct, depending on the actual amplitude of the modulation); adding a 2× pedestal completely destroys their ability to perceive consistent motion from isoluminant displays, reducing their performance to chance. This result is rather different from that reported in an abstract by Zaidi and DeBonet (52). They used a 1× pedestal that may have been insufficient to mask the linear motion component.
To determine whether the inability to perceive the motion direction of pedestaled motion stimuli result might be caused by the low-contrast near-threshold stimuli used for the test, the most sensitive observer in this procedure was tested with pedestaled isoluminant motion stimuli of higher contrasts, right up to the highest contrast available. The observer was equally unable to perceive the motion direction of the 2× pedestaled motion stimulus at all stimulus contrasts. That a 2× pedestal obliterates the perception of motion–direction in isoluminant chromatic stimuli is strikingly different from first- and second-order motion in which a 2× pedestal has no effect whatever on motion–direction thresholds of near-threshold stimuli (30). In second-order motion, even a 7× pedestal (amplitude of pedestal is 7× the amplitude of the motion stimulus) has no effect on motion–direction thresholds (53).
Experiment 3: Interocular vs. Monocular Presentation
Procedure.
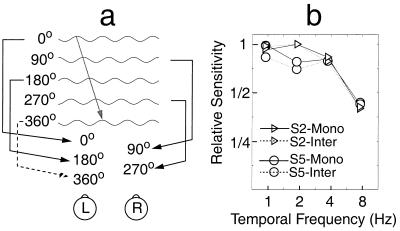
The contrast threshold for discriminating upward vs. downward movement of isoluminant gratings is compared in monocular and interocular displays at four different temporal frequencies: 0.94, 1.88, 3.75, and 7.5 Hz. All of the motion displays consisted of five frames of isoluminant sinewave gratings with a 90-degree phase shift between successive frames (Fig. 1d). In monocular displays, all of the frames were shown to the same eye. In interocular displays (30, 54), the odd frames were shown to one eye, and the even frames were shown to the other eye (Fig. 3a). Because, in interocular displays, successive frames in the same eye are shifted by 180 degrees, there is no motion information in one eye alone. In interocular displays, motion can be extracted only by combining information from the two eyes.
Figure 3.
(a) Interocular display mode; interocular-presentation paradigm. Alternative frames of a five frame display are directed to the left or right eye. From frame-to-frame, there is a 90-degree phase shift to the right. Successive frames in each individual eye have a 180-degree phase shift and therefore contain no motion–direction information. (b) Sensitivity (1/threshold) as a function of temporal frequency in both monocular and interocular display modes for two observers. The sensitivity is normalized for each observer by using the minimum threshold in both monocular and interocular conditions. For both observers, there is no significant difference between the two display modes.
The first phase of the experiment discovered the amplitude of candidate isoluminant gratings that generated 90% correct motion–direction judgment in monocular displays at each of the four temporal frequencies. These 90% correct stimuli were recalibrated with the “improved” dynamic motion calibration (43) and were used to form a set of low-contrast motion stimuli (see below). In the third phase, the method of constant stimuli was used to determine motion–direction thresholds in three display modes (monocular-left, monocular-right, interocular) and four temporal frequencies. Five contrast levels were used in each condition. All trials were intermixed in each session. There were two observers.
Results.
There were no statistically significant differences between the left-eye-only and right-eye-only monocular conditions. Therefore, data from the two monocular display modes were combined. Thresholds for 75% correct motion–direction judgments were estimated from the psychometric functions for each observer at each temporal frequency in both monocular and interocular display modes. Fig. 3b shows contrast sensitivity (1/threshold) as a function of temporal frequency. Each point is based on 200 observations. It is clear that, for both observers, the temporal tuning functions in monocular and interocular display modes overlap almost completely. In other words, the mechanism that computes isoluminant chromatic motion is equally sensitive to monocular and interocular displays; the system is intrinsically binocular.
DISCUSSION
Is It Possible To Generate Isoluminant Motion Stimuli?
The argument against. Green-sensitive and red-sensitive cones occupy different positions in space, and these are not distributed with perfect uniformity, nor are their positions known exactly. Therefore, within any given stimulus, and within any given area, it is statistically impossible to guarantee exactly equal stimulation of red and green cones. Therefore, every stimulus inevitably contains local imperfections and violations of isoluminance. The same argument can be extended to magno and parvo cells or to any other unit of analysis.
Counterargument.
Isoluminance is not a local property but a global property of a stimulus as a whole. That is why each stimulus is calibrated separately and individually. When a stimulus has been calibrated for isoluminance, it remains locally imperfect. However, the sum of all of the local imperfections is insufficient to be useful in generating apparent motion. The calibration procedure demonstrates that the local imperfections cannot combine with a luminance stimulus to produce luminance (first-order) motion. Indeed, with the improved calibration method, even 10× greater imperfections would be insufficient to produce artifactual first-order motion in the isoluminant chromatic displays. We conclude that, although it is impossible to produce a stimulus that has locally perfect isoluminance, the calibration procedure reduces the residual local luminance components to such a low level that they are merely subthreshold luminance noise.
By What Mechanism Is Isoluminant Chromatic Motion Perceived?
The shape of the temporal tuning function, the failure to pass the motion pedestal test, and the indifference to whether frames are presented monocularly or interocularly all suggest that isoluminant chromatic motion is perceived by the third-order motion system (30). Third-order differs from first- and second-order motion mainly in the preprocessing of the visual input before the motion computation. The input to first-order motion processing is the local point contrast c(x, y): the amount, plus or minus, by which the luminance at a point differs from the local mean luminance. [Essentially, c(x, y) is output of center-surround receptive fields, with outputs of ON-center fields taken as positive and outputs of OFF-center fields taken as negative.] The input to second-order motion is the absolute value or perhaps the square of c(x, y), a measure of the total amount of neural activity without regard to sign. The input to second-order motion is the variance of the input to first-order motion. The input to third-order motion is the output of a “salience map,” in which the results of a figure-ground segmentation of the visual field are represented. The salience s(x, y, t) of a point x, y is 1 if, at time t, that point is in an area that is computed to be figure and is 0 if it is in an area that is computed to be ground. Third-order motion can be perceived from alternating-feature displays in which successive frames are completely unrelated, so long as the areas designated as figure move in a consistent direction (33–35). Another characteristic of the third-order motion system is that, although first- and second-order motion are computed monocularly, third-order motion is inherently binocular—it is indifferent to the eye of origin or to alternations in the eye of origin of its input. Indeed, the third-order system cannot process information monocularly before the point of binocular combination (55). The third-order motion system’s temporal half-amplitude frequency typically is 3–4 Hz, only 1/3 the half-amplitude frequency of the first- and second-order motion systems. The third-order system computes motion from all ordinary stimuli that stimulate the first- and second-order motion systems (if they have a sufficiently high amplitude of modulation and sufficiently low spatial and temporal frequencies) as well as from many exotic stimuli (30). And, unlike first- and second-order, third-order motion is strongly influenced by attention (33–35).
Identifying the mechanism for isoluminant motion perception as third-order has enabled us to predict the conditions under which a remarkable motion illusion—motion standstill—would occur. We presume that isoluminant motion of a red–green grating is perceived by the third-order motion system because the red and green stripes in the grating are not equally represented in the salience map. Typically in our gratings, red stripes are perceived as more salient than the green ones. We would suppose that motion is perceived because red is represented as figure and green as ground. Blaser, Sperling, and Lu (35) had already demonstrated that the relative salience of a colored region increases with its saturation. Therefore, in our displays, we increased the saturation of the apparently weaker green component, until motion direction of a moving red–green grating was maximally ambiguous. At this point, we presume the salience modulation is reduced to near zero, so the third-order system computes zero motion, and the perceptual illusion of motion standstill results.
When viewing saturation-balanced stimuli foveally, there was no adjustment that produced complete motion standstill. We interpret this as caused by the uncontrollable influence of attention to either red or green enabling the attended color to be perceived as “figure.” However, by viewing the motion displays parafoveally, 3 degrees peripherally, we were able to find a critical saturation of green (which varied slightly from observer to observer) that produced complete motion standstill for every observer (Z.-L.L., L.A.L., and G.S., unpublished work). One might think that increasing the saturation of color stripes in a grating, thereby making them more distinct, would improve motion perception. That increasing the saturation of the green component of a red–green grating can produce motion standstill certainly demonstrates that the human isoluminant motion computation does not depend on color, per se.¶
That motion standstill occurs with highly saturated red and green stripes proves that there is no motion computation other than the putative third-order motion computation. If there were a color motion computation independent of the third-order motion computation, then a highly saturated red–green grating should be an ideal stimulus, and motion standstill would be impossible.
High-Quality Isoluminant Motion.
In a red–green grating on a white background, both the red and the green stripes have a large representation in the salience map from which third-order motion is computed. When the green stripes in an isoluminant red–green grating are removed, to produce an isoluminant grating consisting only of red stripes on a white background, there is an alternation of salience between high values for red and low values for white. Large modulations of luminance produce good first-order motion stimuli, large modulations of texture-contrast produce good second-order motion stimuli, and large modulations of salience produce good third-order motion stimuli. Thus, when the isoluminant red–white display is moved, a vivid perception of motion results, quite different from the meager perception of motion that is typical of more common isoluminant motion. Recognizing the nature of the third-order motion computation makes it possible to produce the full gamut of motion responses from a moving red–green grating: from motion standstill to easily perceived, smooth motion.
Substituting Other Third-Order Stimuli for Some Isoluminant Frames.
Finally, Blaser et al. (35) provide a demonstration that isoluminant gratings function like third-order stimuli in another context than merely isoluminant chromatic motion. Their observers viewed motion in an alternating feature display very much like the calibration display. The odd frames contained an isoluminant red–green grating. The even frames contained a texture-modulated grating in which areas of high-contrast texture (figure) were interleaved with areas of low-contrast texture (ground). When red and green stripes of the isoluminant grating were of equal saliance, no motion was perceived—for the same reason that no motion is perceived in the motion-standstill demonstration. Increasing the saturation of one of the colors, e.g., red (while maintaining isoluminance), resulted in apparent motion in a consistent direction, the red direction. Selectively attending to red in the previously ambiguous equal-salience display now produced apparent motion in the red direction. Blaser et al. (35) provide a formal model for third-order motion that gives an extremely accurate account of their data and would apply equally well to the present data.
CONCLUSIONS
Taken together, there are five lines of evidence that isoluminant chromatic motion is computed by the third-order motion system and by no other system. (i) The temporal tuning function for isoluminant chromatic motion exactly matches the previously measured tuning function for third-order motion (i.e., low-pass, declining to 1/2 amplitude at 3–6 Hz). (ii) It fails the pedestal test (which is characteristic of third-order motion). (iii) It is perceived as well interocularly as monocularly (which is characteristic of third-order motion). (iv) Motion standstill can be produced with vivid, high saturation gratings, provided the red and green stripes are matched in salience. (v) Isoluminant red–green gratings function like other third-order stimuli in alternating feature displays (perceived by the third-order motion system). That motion standstill can be produced with vivid high-saturation moving color gratings that are nulled for third-order motion means that no other color system is computing the motion of these stimuli. That rules out a specialized chromatic motion mechanism within the color pathway.
Demonstrating that perceiving isoluminant motion is a third-order motion computation does much to resolve the issue of its psychophysical mechanism: The third-order algorithm, as well as its spatial, temporal, and attentional characteristics, has been described quantitatively (30, 35). Because third-order motion has been demonstrated to be sensitive to a wide range of attributes, and because isoluminant chromatic motion is computed by a third-order motion mechanism, we can make the following statement about the physiological basis of isoluminant motion perception. Isoluminant chromatic motion is computed physiologically at a brain level where binocular inputs of form, color, depth, motion, and texture are simultaneously available and where selective attention can exert a major influence.
Acknowledgments
This work was supported by the U.S. Air Force Office of Scientific Research, Life Sciences, Visual Information Processing Program.
Footnotes
A Commentary on this article begins on page 7611.
All displays were shown on an Apple 1710 multisync color monitor (refresh rate = 60 frames/sec) controlled by a 30-bit Radius Thunder 1600/30 graphics card in a 7500/100 PowerPC Macintosh running Matlab programs based on psychtoolbox (36). The monitor was calibrated initially by standard procedures (37) using a Tektronix J17 photometer with a J1820 chromaticity head.
Motion standstill was observed introspectively following the procedures [e.g., type 2 experiments (56)] described in previous reports (1–5). More recent work in progress (Z.-L.L., L.A.L., and G.S., unpublished work) indicates that motion standstill can be measured objectively in experiments that determine the accuracy of motion–direction reports and in which feedback of correctness or incorrectness of a response is provided.
References
- 1.Ramachandran V S, Gregory R L. Nature (London) 1978;275:55–56. doi: 10.1038/275055a0. [DOI] [PubMed] [Google Scholar]
- 2.Moreland J D. In: Color Vision Deficiencies. Verriest V G, editor. Bristol, U.K.: Hilger; 1980. pp. 189–191. [Google Scholar]
- 3.Cavanagh P, Tyler C W, Favreau O E. J Opt Soc Am A. 1984;1:893–899. doi: 10.1364/josaa.1.000893. [DOI] [PubMed] [Google Scholar]
- 4.Livingstone M S, Hubel D H. J Neurosci. 1987;7:3416–3468. doi: 10.1523/JNEUROSCI.07-11-03416.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teller D Y, Lindsey D T. J Opt Soc Am A. 1993;10:1324–1331. doi: 10.1364/josaa.10.001324. [DOI] [PubMed] [Google Scholar]
- 6.Moreland J D. In: ColourDeficiencies IV. Verriest V G, editor. The Hague, Netherlands: Junk; 1982. pp. 61–66. [Google Scholar]
- 7.Moreland J D, Todd D T. In: Normal and Pathologic Color Vision. Marres E, Tost M, Zenker H J, editors. Halle, Germany: Martin Luther Univ. Press; 1987. pp. 39–42. [Google Scholar]
- 8.Mullen K T, Boulton J C. Vision Res. 1992;32:483–488. doi: 10.1016/0042-6989(92)90240-j. [DOI] [PubMed] [Google Scholar]
- 9.Zeki S. Nature (London) 1980;284:412–418. doi: 10.1038/284412a0. [DOI] [PubMed] [Google Scholar]
- 10.Zihl J, von Cramon D, Mai N. Brain. 1983;106:313–340. doi: 10.1093/brain/106.2.313. [DOI] [PubMed] [Google Scholar]
- 11.Livingstone M, Hubel D. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- 12.Zeki S, Watson J D G, Lueck C J, Friston K J, Frackowiak R. J Neurosci. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ansitis S M. Philos Trans R Soc London B. 1980;290:153–168. doi: 10.1098/rstb.1980.0088. [DOI] [PubMed] [Google Scholar]
- 14.Cavanagh P, MacLeod D I, Anstis S M. J Opt Soc Am A. 1987;4:1428–1438. doi: 10.1364/josaa.4.001428. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Stromeyer C F., III J Physiol. 1989;413:563–593. doi: 10.1113/jphysiol.1989.sp017669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer J, Mobley L A, Teller D Y. J Opt Soc Am A. 1993;10:1353–1362. doi: 10.1364/josaa.10.001353. [DOI] [PubMed] [Google Scholar]
- 17.Albright T D. Trends Neurosci. 1991;14:266–269. doi: 10.1016/0166-2236(91)90134-g. [DOI] [PubMed] [Google Scholar]
- 18.Cavanagh P. Science. 1992;257:1563–1565. doi: 10.1126/science.1523411. [DOI] [PubMed] [Google Scholar]
- 19.Gorea A, Papathomas T V, Kovacs I. Proc Natl Acad Sci USA. 1993;90:11197–11201. doi: 10.1073/pnas.90.23.11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Culham J C, Cavanagh P. Vision Res. 1994;34:2701–2706. doi: 10.1016/0042-6989(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 21.Gegenfurtner K R, Hawken M J. Trends Neurosci. 1996;19:394–401. doi: 10.1016/S0166-2236(96)10036-9. [DOI] [PubMed] [Google Scholar]
- 22.Cavanagh P, Favreau O E. Vision Res. 1985;25:1595–1601. doi: 10.1016/0042-6989(85)90129-4. [DOI] [PubMed] [Google Scholar]
- 23.Derrington A M, Badcock D R. Vision Res. 1985;25:1879–1884. doi: 10.1016/0042-6989(85)90011-2. [DOI] [PubMed] [Google Scholar]
- 24.Krauskopf J, Farell B. Nature (London) 1990;348:328–331. doi: 10.1038/348328a0. [DOI] [PubMed] [Google Scholar]
- 25.Papathomas T V, Gorea A, Julesz B. Vision Res. 1991;31:1883–1891. doi: 10.1016/0042-6989(91)90183-6. [DOI] [PubMed] [Google Scholar]
- 26.Kooi F L, de Valois K K. Vision Res. 1992;32:657–668. doi: 10.1016/0042-6989(92)90182-i. [DOI] [PubMed] [Google Scholar]
- 27.Chichilnisky E-J, Heeger D, Wandell B A. Vision Res. 1993;33:2113–2125. doi: 10.1016/0042-6989(93)90010-t. [DOI] [PubMed] [Google Scholar]
- 28.Cropper S J, Derrington A M. Nature (London) 1996;379:72–74. doi: 10.1038/379072a0. [DOI] [PubMed] [Google Scholar]
- 29.Webster M A, Mollon J D. Vision Res. 1997;37:1479–1498. doi: 10.1016/s0042-6989(96)00289-1. [DOI] [PubMed] [Google Scholar]
- 30.Lu Z-L, Sperling G. Vision Res. 1995;35:2697–2722. doi: 10.1016/0042-6989(95)00025-u. [DOI] [PubMed] [Google Scholar]
- 31.Lu Z-L, Sperling G. Curr Dir Psychol Sci. 1996;5:44–53. [Google Scholar]
- 32.Sperling, G. & Lu, Z.-L. (1998) Invest. Ophthalmol. Vis. Sci.39, Suppl., 461.
- 33.Lu Z-L, Sperling G. Nature (London) 1995;377:237–239. doi: 10.1038/377237a0. [DOI] [PubMed] [Google Scholar]
- 34.Sperling G, Lu Z-L. In: High-level Motion Processing. Watanabe T, editor. Cambridge, MA: MIT Press; 1998. pp. 153–183. [Google Scholar]
- 35.Blaser, E., Sperling, G. & Lu, Z.-L. (1998) Invest. Ophthalmol. Vis. Sci.38, Suppl., 687.
- 36.Brainard D. Spatial Vis. 1997;10:443–446. [Google Scholar]
- 37.Brainard D. In: Human Color Vision, Appendix IV. Kaiser P K, Boynton R M, editors. Washington, DC: Opt. Soc. Am.; 1996. pp. 563–579. [Google Scholar]
- 38.MacLeod D I A, Boynton R M. J Opt Soc Am A. 1979;69:1183–1186. doi: 10.1364/josa.69.001183. [DOI] [PubMed] [Google Scholar]
- 39.Derrington A M, Krauskopf J, Lennie P. J Physiol (London) 1984;357:241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howarth P A. Ophthalmic Physiol Opt. 1984;4:223–226. doi: 10.1111/j.1475-1313.1984.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 41.Simonet P, Campbell M C. Ophthalmic Physiol Opt. 1990;10:271–279. [PubMed] [Google Scholar]
- 42.Zhang X, Bradley A, Thibos L N. J Opt Soc Am A. 1993;10:213–220. doi: 10.1364/josaa.10.000213. [DOI] [PubMed] [Google Scholar]
- 43.Lu, Z.-L. & Sperling, G. (1998) Invest. Ophthalmol. Visual Sci.40, Suppl., S199.
- 44.Anstis S, Cavanagh P. In: Color Vision. Mollon J D, Sharpe E T, editors. New York: Academic; 1983. pp. 155–166. [Google Scholar]
- 45.van Santen J P, Sperling G. J Opt Soc Am A. 1984;1:451–473. doi: 10.1364/josaa.1.000451. [DOI] [PubMed] [Google Scholar]
- 46.Werkhoven P, Sperling G, Chubb C. Vision Res. 1993;33:463–485. doi: 10.1016/0042-6989(93)90253-s. [DOI] [PubMed] [Google Scholar]
- 47.Derrington A M, Henning G B. Vision Res. 1993;33:799–811. doi: 10.1016/0042-6989(93)90199-7. [DOI] [PubMed] [Google Scholar]
- 48.Pantle A, Picciano L. Science. 1976;193:500–502. doi: 10.1126/science.941023. [DOI] [PubMed] [Google Scholar]
- 49.Petersik J T, Hicks K I, Pantle A J. Perception. 1978;7:371–383. doi: 10.1068/p070371. [DOI] [PubMed] [Google Scholar]
- 50.Julesz B, Payne R. Vision Res. 1968;8:433–444. doi: 10.1016/0042-6989(68)90111-9. [DOI] [PubMed] [Google Scholar]
- 51.Zanker J M. Vision Res. 1993;33:553–569. doi: 10.1016/0042-6989(93)90258-x. [DOI] [PubMed] [Google Scholar]
- 52.Zaidi Q, DeBonet J. OSA Annual Meeting Program. Washington, DC: Optical Society of America; 1994. p. 99. [Google Scholar]
- 53.Lu Z-L, Sperling G. J Opt Soc Am A. 1996;13:2305–2318. doi: 10.1364/josaa.13.002305. [DOI] [PubMed] [Google Scholar]
- 54.Shadlen M, Carney T. Science. 1986;232:95–97. doi: 10.1126/science.3952502. [DOI] [PubMed] [Google Scholar]
- 55.Solomon, J. A. & Morgan, M. J. (1999) Vision Res., in press.
- 56.Sperling G, Dosher B A, Landy M S. J Exp Psychol Human Perception Performance. 1990;16:445–450. [PubMed] [Google Scholar]