Abstract
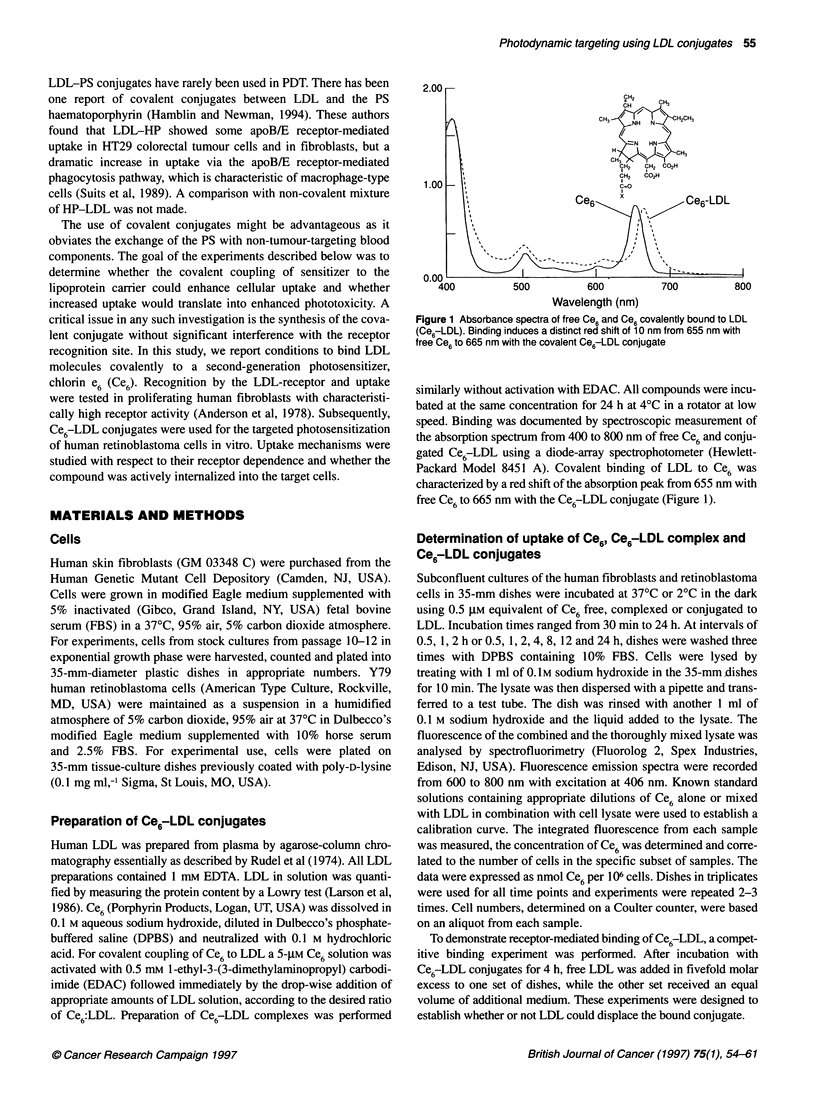
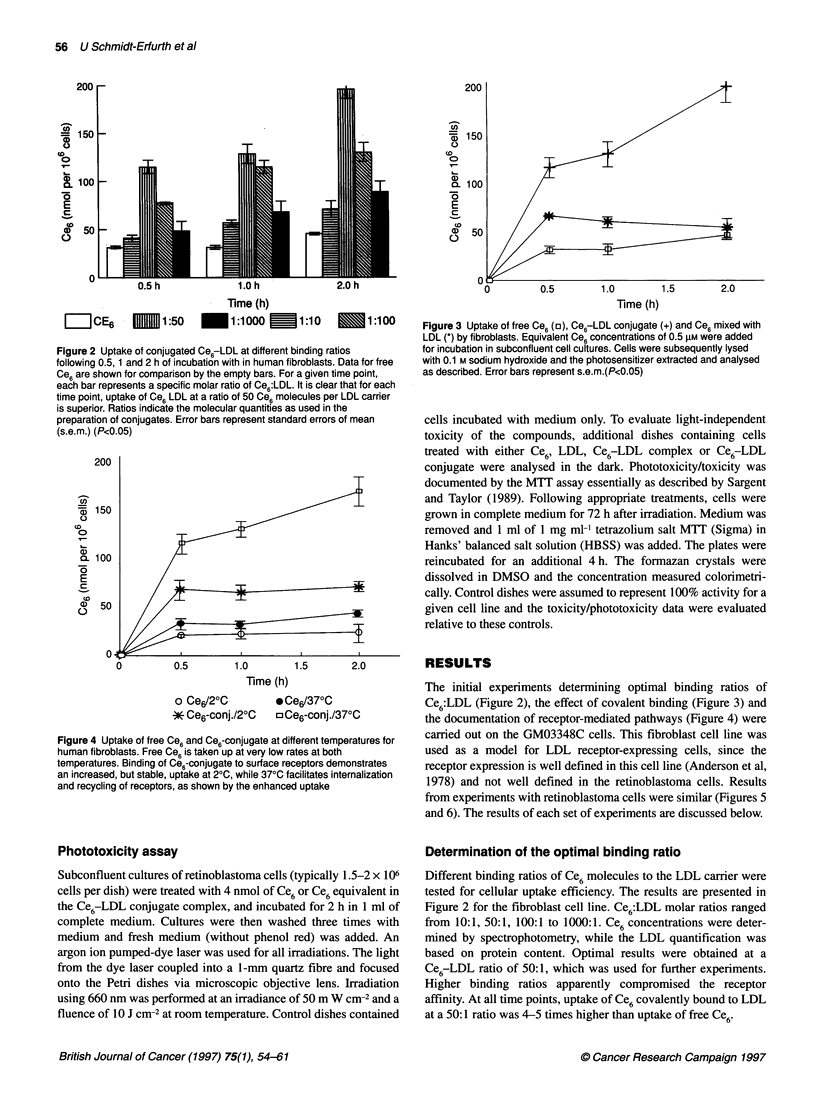
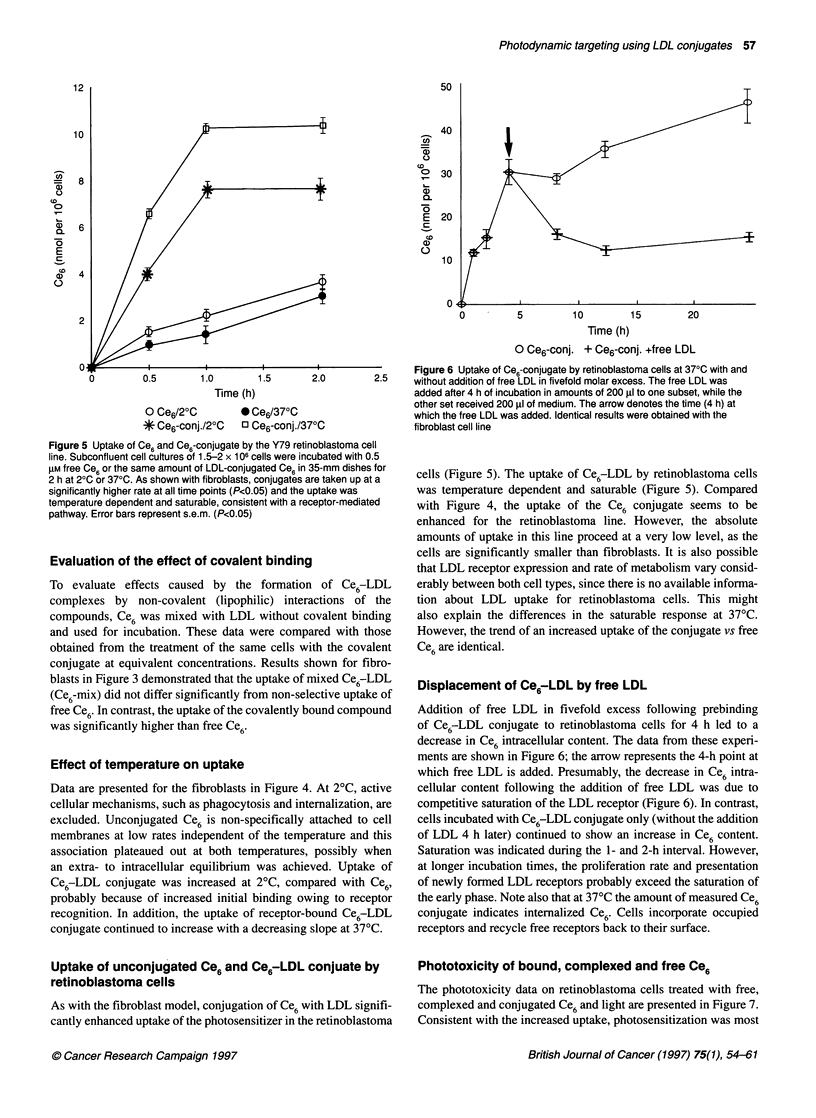
Combination of photosensitizers with carrier molecules has been shown to enhance the efficiency of photodynamic therapy (PDT). Owing to an increased expression of their receptors on some malignant and proliferating cells, low-density lipoproteins (LDLs) are potential endogenous carriers. A photosensitizer, chlorin e6 (Ce6), was covalently bound to LDL via carbodiimide activation. The Ce6-LDL conjugate was evaluated on a fibroblast cell line with defined LDL receptor expression and a retinoblastoma cell line (Y79). Uptake of free Ce6 and Ce6 either covalently bound to or complexed with LDL was measured by spectrofluorimetry. Phototoxicity after irradiation at 660 nm was determined by a mitochondrial activity assay (MTT). Covalent binding to LDL significantly increased the uptake of Ce6 for both cell lines by a factor of 4-5. A Ce6: LDL binding ratio of 50:1 was optimal. A receptor-mediated uptake was demonstrated by saturability and competitive inhibition by free LDL. Binding also occurred at 2 degrees C and was attributed to non-specific associations. Irradiation with 10 J cm-2 of 660 nm light after treatment of cells with Ce6-LDL conjugate reduced the MTT activity by 80%, while free or mixed Ce6 induced a maximum of 10% reduction in the MTT activity following identical treatment conditions. These data suggest that targeting of LDL receptor-bearing cells using covalently bound carriers, such as LDL, might increase the efficiency and selectivity of PDT. Intraocular tumours such as retinoblastomas could be appropriate targets for such an approach owing to the ease of access of light sources and the need for non-invasive approaches in sensitive ocular sites.
Full text
PDF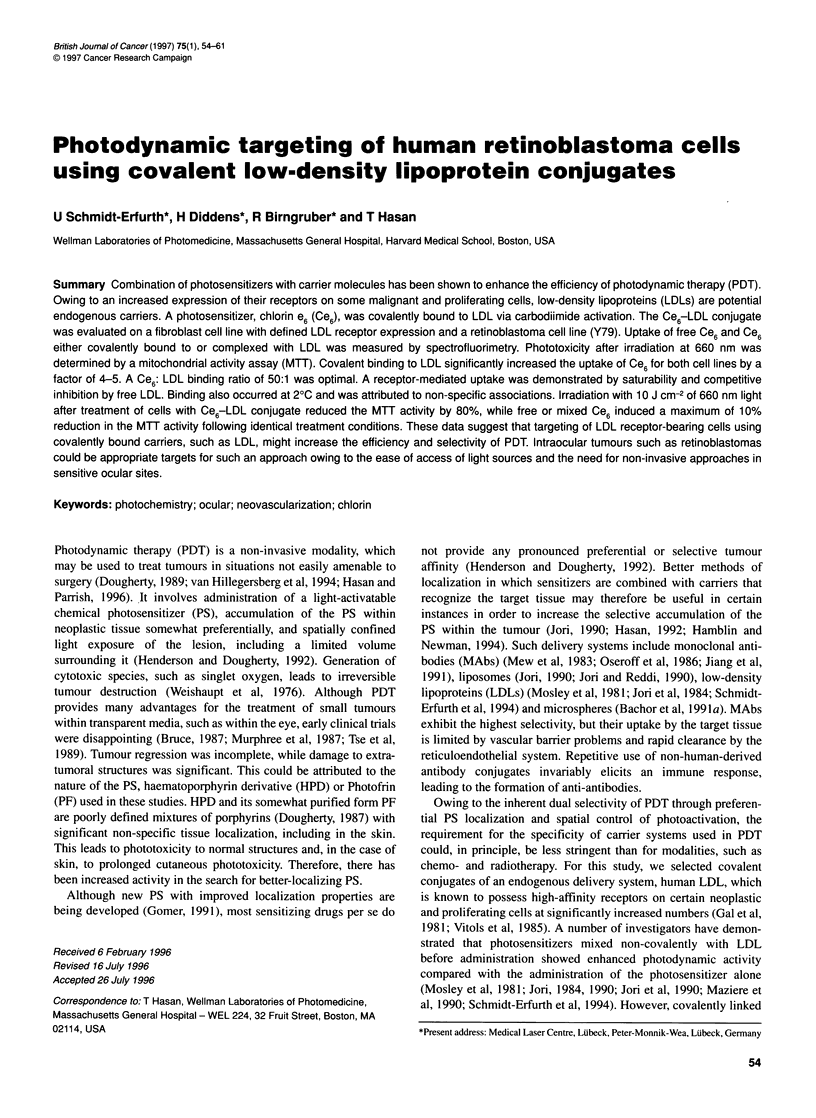



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson D. H., Jereb B., Ellsworth R. M. External beam radiation for retinoblastoma. Bull N Y Acad Med. 1981 Nov;57(9):787–803. [PMC free article] [PubMed] [Google Scholar]
- Allison B. A., Pritchard P. H., Levy J. G. Evidence for low-density lipoprotein receptor-mediated uptake of benzoporphyrin derivative. Br J Cancer. 1994 May;69(5):833–839. doi: 10.1038/bjc.1994.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison B. A., Pritchard P. H., Richter A. M., Levy J. G. The plasma distribution of benzoporphyrin derivative and the effects of plasma lipoproteins on its biodistribution. Photochem Photobiol. 1990 Sep;52(3):501–507. doi: 10.1111/j.1751-1097.1990.tb01792.x. [DOI] [PubMed] [Google Scholar]
- Anderson R. G., Vasile E., Mello R. J., Brown M. S., Goldstein J. L. Immunocytochemical visualization of coated pits and vesicles in human fibroblasts: relation to low density lipoprotein receptor distribution. Cell. 1978 Nov;15(3):919–933. doi: 10.1016/0092-8674(78)90276-3. [DOI] [PubMed] [Google Scholar]
- Bachor R., Shea C. R., Gillies R., Hasan T. Photosensitized destruction of human bladder carcinoma cells treated with chlorin e6-conjugated microspheres. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1580–1584. doi: 10.1073/pnas.88.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Regulation of the activity of the low density lipoprotein receptor in human fibroblasts. Cell. 1975 Nov;6(3):307–316. doi: 10.1016/0092-8674(75)90182-8. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Photodynamic therapy--new approaches. Semin Surg Oncol. 1989;5(1):6–16. doi: 10.1002/ssu.2980050104. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Photosensitizers: therapy and detection of malignant tumors. Photochem Photobiol. 1987 Jun;45(6):879–889. doi: 10.1111/j.1751-1097.1987.tb07898.x. [DOI] [PubMed] [Google Scholar]
- Endemann G., Stanton L. W., Madden K. S., Bryant C. M., White R. T., Protter A. A. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993 Jun 5;268(16):11811–11816. [PubMed] [Google Scholar]
- Gal D., MacDonald P. C., Porter J. C., Simpson E. R. Cholesterol metabolism in cancer cells in monolayer culture. III. Low-density lipoprotein metabolism. Int J Cancer. 1981 Sep 15;28(3):315–319. doi: 10.1002/ijc.2910280310. [DOI] [PubMed] [Google Scholar]
- Goff B. A., Bamberg M., Hasan T. Photoimmunotherapy of human ovarian carcinoma cells ex vivo. Cancer Res. 1991 Sep 15;51(18):4762–4767. [PubMed] [Google Scholar]
- Goff B. A., Hermanto U., Rumbaugh J., Blake J., Bamberg M., Hasan T. Photoimmunotherapy and biodistribution with an OC125-chlorin immunoconjugate in an in vivo murine ovarian cancer model. Br J Cancer. 1994 Sep;70(3):474–480. doi: 10.1038/bjc.1994.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomer C. J. Preclinical examination of first and second generation photosensitizers used in photodynamic therapy. Photochem Photobiol. 1991 Dec;54(6):1093–1107. doi: 10.1111/j.1751-1097.1991.tb02133.x. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Olch C. L., Folgelman A. M. Role of lysines in mediating interaction of modified low density lipoproteins with the scavenger receptor of human monocyte macrophages. J Biol Chem. 1984 Sep 25;259(18):11305–11311. [PubMed] [Google Scholar]
- Hamblin M. R., Newman E. L. Photosensitizer targeting in photodynamic therapy. II. Conjugates of haematoporphyrin with serum lipoproteins. J Photochem Photobiol B. 1994 Nov;26(2):147–157. doi: 10.1016/1011-1344(94)07036-9. [DOI] [PubMed] [Google Scholar]
- Henderson B. W., Fingar V. H. Relationship of tumor hypoxia and response to photodynamic treatment in an experimental mouse tumor. Cancer Res. 1987 Jun 15;47(12):3110–3114. [PubMed] [Google Scholar]
- Itakura H., Matsumoto A., Asaoka H., Kodama T. [Structure and function of the scavenger receptor]. Nihon Rinsho. 1993 Apr;51(4):1083–1091. [PubMed] [Google Scholar]
- Jiang F. N., Liu D. J., Neyndorff H., Chester M., Jiang S. Y., Levy J. G. Photodynamic killing of human squamous cell carcinoma cells using a monoclonal antibody-photosensitizer conjugate. J Natl Cancer Inst. 1991 Sep 4;83(17):1218–1225. doi: 10.1093/jnci/83.17.1218. [DOI] [PubMed] [Google Scholar]
- Jori G., Beltramini M., Reddi E., Salvato B., Pagnan A., Ziron L., Tomio L., Tsanov T. Evidence for a major role of plasma lipoproteins as hematoporphyrin carriers in vivo. Cancer Lett. 1984 Oct;24(3):291–297. doi: 10.1016/0304-3835(84)90025-9. [DOI] [PubMed] [Google Scholar]
- Khoo J. C., Miller E., McLoughlin P., Steinberg D. Enhanced macrophage uptake of low density lipoprotein after self-aggregation. Arteriosclerosis. 1988 Jul-Aug;8(4):348–358. doi: 10.1161/01.atv.8.4.348. [DOI] [PubMed] [Google Scholar]
- Larson E., Howlett B., Jagendorf A. Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem. 1986 Jun;155(2):243–248. doi: 10.1016/0003-2697(86)90432-x. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Scherer P. E., Vidugiriene J., Tang Z., Hermanowski-Vosatka A., Tu Y. H., Cook R. F., Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994 Jul;126(1):111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziere J. C., Santus R., Morliere P., Reyftmann J. P., Candide C., Mora L., Salmon S., Maziere C., Gatt S., Dubertret L. Cellular uptake and photosensitizing properties of anticancer porphyrins in cell membranes and low and high density lipoproteins. J Photochem Photobiol B. 1990 Jun;6(1-2):61–68. doi: 10.1016/1011-1344(90)85074-7. [DOI] [PubMed] [Google Scholar]
- Mew D., Wat C. K., Towers G. H., Levy J. G. Photoimmunotherapy: treatment of animal tumors with tumor-specific monoclonal antibody-hematoporphyrin conjugates. J Immunol. 1983 Mar;130(3):1473–1477. [PubMed] [Google Scholar]
- Moan J., Peng Q., Evensen J. F., Berg K., Western A., Rimington C. Photosensitizing efficiencies, tumor- and cellular uptake of different photosensitizing drugs relevant for photodynamic therapy of cancer. Photochem Photobiol. 1987 Nov;46(5):713–721. doi: 10.1111/j.1751-1097.1987.tb04837.x. [DOI] [PubMed] [Google Scholar]
- Mosley S. T., Goldstein J. L., Brown M. S., Falck J. R., Anderson R. G. Targeted killing of cultured cells by receptor-dependent photosensitization. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5717–5721. doi: 10.1073/pnas.78.9.5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphree A. L., Cote M., Gomer C. J. The evolution of photodynamic therapy techniques in the treatment of intraocular tumors. Photochem Photobiol. 1987 Nov;46(5):919–923. doi: 10.1111/j.1751-1097.1987.tb04869.x. [DOI] [PubMed] [Google Scholar]
- Murphree A. L., Cote M., Gomer C. J. The evolution of photodynamic therapy techniques in the treatment of intraocular tumors. Photochem Photobiol. 1987 Nov;46(5):919–923. doi: 10.1111/j.1751-1097.1987.tb04869.x. [DOI] [PubMed] [Google Scholar]
- Naito M., Suzuki H., Mori T., Matsumoto A., Kodama T., Takahashi K. Coexpression of type I and type II human macrophage scavenger receptors in macrophages of various organs and foam cells in atherosclerotic lesions. Am J Pathol. 1992 Sep;141(3):591–599. [PMC free article] [PubMed] [Google Scholar]
- Oseroff A. R., Ohuoha D., Hasan T., Bommer J. C., Yarmush M. L. Antibody-targeted photolysis: selective photodestruction of human T-cell leukemia cells using monoclonal antibody-chlorin e6 conjugates. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8744–8748. doi: 10.1073/pnas.83.22.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S. Novel atherogenic, oxidative modification of low-density lipoprotein. Diabetes Metab Rev. 1991 Sep;7(3):163–171. doi: 10.1002/dmr.5610070305. [DOI] [PubMed] [Google Scholar]
- Roberts W. G., Hasan T. Role of neovasculature and vascular permeability on the tumor retention of photodynamic agents. Cancer Res. 1992 Feb 15;52(4):924–930. [PubMed] [Google Scholar]
- Rudel L. L., Lee J. A., Morris M. D., Felts J. M. Characterization of plasma lipoproteins separated and purified by agarose-column chromatography. Biochem J. 1974 Apr;139(1):89–95. doi: 10.1042/bj1390089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudling M. J., Angelin B., Peterson C. O., Collins V. P. Low density lipoprotein receptor activity in human intracranial tumors and its relation to the cholesterol requirement. Cancer Res. 1990 Feb 1;50(3):483–487. [PubMed] [Google Scholar]
- Rutledge J. C., Curry F. R., Lenz J. F., Davis P. A. Low density lipoprotein transport across a microvascular endothelial barrier after permeability is increased. Circ Res. 1990 Feb;66(2):486–495. doi: 10.1161/01.res.66.2.486. [DOI] [PubMed] [Google Scholar]
- Sargent J. M., Taylor C. G. Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Br J Cancer. 1989 Aug;60(2):206–210. doi: 10.1038/bjc.1989.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Erfurth U., Bauman W., Gragoudas E., Flotte T. J., Michaud N. A., Birngruber R., Hasan T. Photodynamic therapy of experimental choroidal melanoma using lipoprotein-delivered benzoporphyrin. Ophthalmology. 1994 Jan;101(1):89–99. doi: 10.1016/s0161-6420(13)31242-1. [DOI] [PubMed] [Google Scholar]
- Schmidt-Erfurth U., Hasan T., Schomacker K., Flotte T., Birngruber R. In vivo uptake of liposomal benzoporphyrin derivative and photothrombosis in experimental corneal neovascularization. Lasers Surg Med. 1995;17(2):178–188. doi: 10.1002/lsm.1900170207. [DOI] [PubMed] [Google Scholar]
- Sery T. W. Photodynamic killing of retinoblastoma cells with hematoporphyrin and light. Cancer Res. 1979 Jan;39(1):96–100. [PubMed] [Google Scholar]
- Sery T. W., Shields J. A., Augsburger J. J., Shah H. G. Photodynamic therapy of human ocular cancer. Ophthalmic Surg. 1987 Jun;18(6):413–418. [PubMed] [Google Scholar]
- Shields C. L., Shields J. A., De Potter P., Minelli S., Hernandez C., Brady L. W., Cater J. R. Plaque radiotherapy in the management of retinoblastoma. Use as a primary and secondary treatment. Ophthalmology. 1993 Feb;100(2):216–224. doi: 10.1016/s0161-6420(93)31667-2. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M. Cellular interactions of lipoproteins with the vascular endothelium: endocytosis and transcytosis. Targeted Diagn Ther. 1991;5:45–95. doi: 10.1201/9780203748831-3. [DOI] [PubMed] [Google Scholar]
- Suits A. G., Chait A., Aviram M., Heinecke J. W. Phagocytosis of aggregated lipoprotein by macrophages: low density lipoprotein receptor-dependent foam-cell formation. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2713–2717. doi: 10.1073/pnas.86.8.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tertov V. V., Sobenin I. A., Gabbasov Z. A., Popov E. G., Orekhov A. N. Lipoprotein aggregation as an essential condition of intracellular lipid accumulation caused by modified low density lipoproteins. Biochem Biophys Res Commun. 1989 Aug 30;163(1):489–494. doi: 10.1016/0006-291x(89)92163-3. [DOI] [PubMed] [Google Scholar]
- Tse D. T., Dutton J. J., Weingeist T. A., Hermsen V. M., Kersten R. C. Hematoporphyrin photoradiation therapy for intraocular and orbital malignant melanoma. Arch Ophthalmol. 1984 Jun;102(6):833–838. doi: 10.1001/archopht.1984.01040030653011. [DOI] [PubMed] [Google Scholar]
- Vasile E., Simionescu M., Simionescu N. Visualization of the binding, endocytosis, and transcytosis of low-density lipoprotein in the arterial endothelium in situ. J Cell Biol. 1983 Jun;96(6):1677–1689. doi: 10.1083/jcb.96.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitols S., Gahrton G., Björkholm M., Peterson C. Hypocholesterolaemia in malignancy due to elevated low-density-lipoprotein-receptor activity in tumour cells: evidence from studies in patients with leukaemia. Lancet. 1985 Nov 23;2(8465):1150–1154. doi: 10.1016/s0140-6736(85)92679-0. [DOI] [PubMed] [Google Scholar]
- Vitols S. Uptake of low-density lipoprotein by malignant cells--possible therapeutic applications. Cancer Cells. 1991 Dec;3(12):488–495. [PubMed] [Google Scholar]
- Vlodavsky I., Fielding P. E., Fielding C. J., Gospodarowicz D. Role of contact inhibition in the regulation of receptor-mediated uptake of low density lipoprotein in cultured vascular endothelial cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):356–360. doi: 10.1073/pnas.75.1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaupt K. R., Gomer C. J., Dougherty T. J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976 Jul;36(7 Pt 1):2326–2329. [PubMed] [Google Scholar]
- Winther J. Photodynamic therapy effect in an intraocular retinoblastoma-like tumour assessed by an in vivo to in vitro colony forming assay. Br J Cancer. 1989 Jun;59(6):869–872. doi: 10.1038/bjc.1989.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C. N., Milanesi C., Jori G. An ultrastructural comparative evaluation of tumors photosensitized by porphyrins administered in aqueous solution, bound to liposomes or to lipoproteins. Photochem Photobiol. 1988 Oct;48(4):487–492. doi: 10.1111/j.1751-1097.1988.tb02850.x. [DOI] [PubMed] [Google Scholar]
- van Hillegersberg R., Kort W. J., Wilson J. H. Current status of photodynamic therapy in oncology. Drugs. 1994 Oct;48(4):510–527. doi: 10.2165/00003495-199448040-00003. [DOI] [PubMed] [Google Scholar]



