Abstract
Bone morphogenetic protein-15 (BMP-15) is an oocyte-secreted factor critical for the regulation of ovarian physiology. When recombinant human BMP-15 (rhBMP-15) produced in human embryonic kidney 293 cells was subjected to SDS-PAGE analysis, two mature protein forms corresponding to 16 kDa (P16) and 17 kDa (P17) were observed. Despite the physiological relevance and critical function of BMP-15 in female reproduction, little is known about the structure of rhBMP-15. Here, we have analyzed the structure of the rhBMP-15 mature proteins (P16 and P17) using state-of-the-art proteomics technology. Our findings are as follows: (1) the N-terminal amino acid of P16 and P17 is pyroglutamic acid; (2) the Ser residue at the sixth position of P16 is phosphorylated; (3) P17 is O-glycosylated at Thr10; and (4) the C-terminal amino acid of P16 and P17 is truncated. These findings are the first knowledge of the structure of rhBMP-15 mature protein toward understanding the molecular basis of BMP-15 function and could provide an important contribution to the rapidly progressing research area involving oocyte-specific growth factors in modulation of female fertility.
Keywords: bone morphogenetic protein, post-translational modification, mass spectrometry, phosphorylation, neutral loss scan, O-glycosylation, proteomics analysis, pyroglutamic acid
Much of the recent research in the field of ovarian physiology has been focused on characterizing the cell-to-cell communication, especially between oocytes and follicular somatic cells (Eppig 2001). The current theory is that some oocyte factors act as morphogens to control follicle growth and differentiation (Erickson and Shimasaki 2000). Compelling evidence that this concept operates in vertebrates comes from the important work of Matzuk and co-workers (Dong et al. 1996; Carabatsos et al. 1998; Elvin et al. 2000), demonstrating that oocyte-derived growth and differentiation factor-9 (GDF-9), a member of the transforming growth factor-β (TGF-β) superfamily, is critical for normal folliculogenesis and female fertility.
In 1998, another oocyte-specific growth factor, bone morphogenetic protein-15 (BMP-15) (Dube et al. 1998) or GDF-9B (Laitinen et al. 1998), was discovered as a new member of the TGF-β superfamily. The primary structure of mouse, human, and rat BMP-15 has high sequence homology with the corresponding sequences of GDF-9 in these species (McPherron and Lee 1993; McGrath et al. 1995; Dube et al. 1998; Hayashi et al. 1999; Jaatinen et al. 1999). An interesting structural feature of BMP-15 and GDF-9 is that they lack the fourth cysteine residue of the seven cysteines that are typically conserved in most members of the TGF-β superfamily. Since the fourth cysteine is responsible for inter-subunit disulfide bond formation, it was postulated that BMP-15 and GDF-9 may exist as monomers or as noncovalently linked hetero- and/or homodimers. Our studies using recombinant human BMP-15 (rhBMP-15) and rhGDF-9 have demonstrated that both rhBMP-15 and rhGDF-9 form noncovalently linked homodimers and, when co-expressed, can also form heterodimers (Liao et al. 2003).
In the past several years, we have extensively studied the biological functions of rhBMP-15 (Shimasaki et al. 2004). Briefly, using rat granulosa cells (GCs) from early antral follicles, we have shown that BMP-15 is a stimulator of GC mitosis and proliferation (Otsuka et al. 2000). In GCs, BMP-15 also stimulates the mRNA expression of kit ligand, a factor that is necessary for early follicle growth (Otsuka and Shimasaki 2002). In this capacity, BMP-15 acts as part of a gamete–somatic cell negative-feedback loop between oocytes and GCs in which BMP-15 promotes the expression of kit ligand in GCs, which, in turn, acts to suppress the expression of oocyte BMP-15. BMP-15 is also potent in suppressing FSH receptor mRNA expression in GCs (Otsuka et al. 2001). Accordingly, the FSH-induced mRNA expression of steroidogenic acute regulatory protein, P450 side-chain cleavage enzyme, 3β-hydroxysteroid dehydrogenase, LH receptor, and inhibin/activin subunits (α, βA, and βB) are also inhibited by BMP-15. We have also demonstrated that BMP-15 stimulates cumulus expansion (Yoshino et al. 2006), which is associated with the enhanced expression of epidermal growth factor-like growth factors in cumulus cells as well as a series of the downstream molecules, including cyclooxygenase-2, hyaluronan synthase-2, tumor necrosis factor-stimulated gene-6, and pentraxin-3, all of which are necessary for normal cumulus expansion.
The biological studies of BMP-15 described above have been performed using rhBMP-15 tagged with a Flag epitope at the C terminus that was purified from conditioned medium of the transfected human embryonic kidney (HEK) 293 cells using an anti-Flag monoclonal antibody (mAb) agarose column. SDS–polyacrylamide gel electrophoresis (PAGE) analysis of the immunopurified proteins showed two distinct mature proteins (16 and 17 kDa) (Otsuka et al. 2000). However, the nature of these mature proteins is unknown, despite the established physiological relevance of BMP-15 in ovarian function. To fill in this gap in our knowledge, herein we have characterized the structure of rhBMP-15 mature protein.
Results
SDS-PAGE analysis
As reported previously (Otsuka et al. 2000), SDS-PAGE analysis of the purified rhBMP-15 tagged with a Flag epitope at the C terminus showed two distinct mature protein bands migrating at 16 and 17 kDa (Fig. 1A). In addition, larger proteins migrating at 33 and 50 kDa were detected (Fig. 1A). Although these proteins were all purified using anti-Flag mAb agarose, the 33-kDa protein was not detected by anti-Flag mAb (Fig. 1B), indicating that it lacks the Flag epitope. Based on the molecular weight, these data suggest that the 33- and 50-kDa proteins are most likely the pro-region and pro-protein of rhBMP-15, respectively. Therefore, it seems that the pro-region protein of rhBMP-15 remains associated with the mature protein after cleavage, similar to other members of the TGF-β superfamily, such as TGF-β-1, -2, and -3, BMP-7, and GDF-8 (Thies et al. 2000; Lee and McPherron 2001; Jiang et al. 2004; Gregory et al. 2005). In the current study, we have focused on characterizing the structure of the functional mature proteins (P16 and P17).
Figure 1.
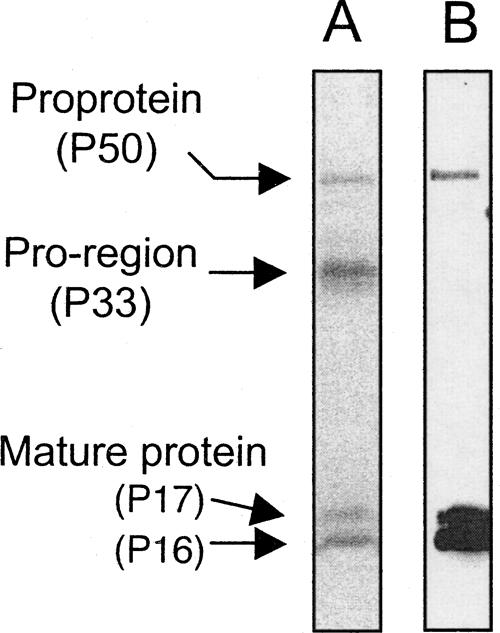
(A) SDS-PAGE analysis of purified rhBMP-15 protein. rhBMP-15 purified by anti-Flag mAb agarose affinity column chromatography was separated on 12% SDS-PAGE and stained by SYPRO Ruby. (B) The same samples were subjected to Western immunoblotting using anti-Flag mAb.
N-Terminal amino acid sequence analysis of P16 and P17
After separation of P16 and P17 by SDS-PAGE, the N-terminal amino acid sequence of these proteins was analyzed. However, despite considerable effort, no phenylthiohydantoin (PTH)-amino acids were released from either protein by Edman degradation reaction, indicating that their amino termini (Gln at amino acid #268, GenBank accession numbers AF082349 and AF082350) might be blocked. Because the N-terminal Gln or Glu are known to form pyro-Glu under aqueous conditions, we treated the P16 and P17 proteins with pyroglutamate aminopeptidase. Consequently, the treated proteins exhibited the N-terminal sequence of Ala-Asp-Gly-Ile-Ser-Ala-Glu-Val-Thr-Ala-, which is identical to that located at amino acids #269–278. Therefore, we concluded that the N-terminal amino acid of the rhBMP-15 mature proteins (both P16 and P17) is pyro-Glu instead of Gln.
MALDI-TOF MS analysis of P16 and P17
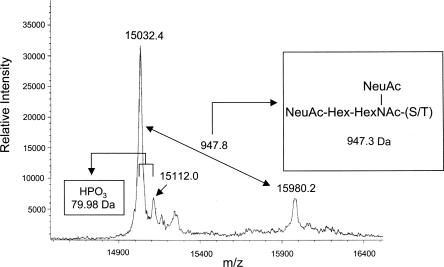
We next performed a further analysis of the rhBMP-15 mature protein using the MALDI-TOF MS technique. As shown in Figure 2, the MALDI-TOF MS analysis revealed a major peak at m/z 15,032.41+. This molecular mass (MM, 15,032.4 Da) is larger than the calculated MM (15,162.0 Da) for a predicted protein having the N-terminal pyro-Glu and the C-terminal Flag-epitope. Given the fact that the C-terminal Lys and Arg are often truncated in proteins produced by mammalian cell cultures (Harris 1995), we recalculated the MM without the C-terminal Lys of the Flag-epitope. When this was done, the calculated MM was 15,033.8 Da, which differs by only 1.4 Da from the observed MM (15,032.4 Da). This difference is much less than the maximum error (0.1%, or 15 Da in this sample) predicted by the manufacturer.
Figure 2.
MALDI-TOF MS spectrum of P16 and P17. Ion relative intensities are shown on the Y-axis, and ion masses (in m/z) are shown on the X-axis.
The second-largest peak is at m/z 15,980.21+ (Fig. 2). Thus, this peak and the major peak at m/z 15,032.41+ appear to correspond to the P17 and P16 proteins, respectively. The difference in MM between these two peaks is 947.8 Da. The computer analysis of this difference indicated the presence of a mucin-type O-glycan with the structure of {NeuAc(2)HexHexNAc} in the m/z 15,032.41+ protein. The theoretical MM of this addition is 947.3 Da, which is almost identical to the observed MM difference (947.8 Da).
In addition to these two peaks, we have also detected a peak at m/z 15,112.01+ (Fig. 2). The MM of this peak is 79.6 Da greater than that of the largest peak (m/z 15,032.41+), suggesting the presence of a single phosphorylation (79.98 Da) in the P16 protein.
Identification of the O-linked oligosaccharide structure and its position in P17
To confirm the structure of the predicted mucin-type O-glycan {NeuAc(2)HexHexNAc} present in P17 and to determine its position, the P17 protein separated by SDS-PAGE was subjected to in-gel digestion with trypsin, and the resulting tryptic peptides were analyzed by the LC-MS/MS neutral loss scanning method. For this purpose, we first attempted to detect the tryptic fragment bearing an O-glycan by scanning intense oligosaccharide-derived fragment ions of m/z 204 (HexNAc), 366 (HexHexNAc), 163 (Hex), and 292 (Neu5Ac). However, we could not detect any O-glycosylated peptides in the P17 tryptic peptides. Therefore, we employed a computational prediction program, NetOGlyc 3.1 Server (Julenius et al. 2005), to predict the site of O-glycan in human BMP-15. This computational analysis suggested Ser6 and Thr10 as putative O-glycosylation sites.
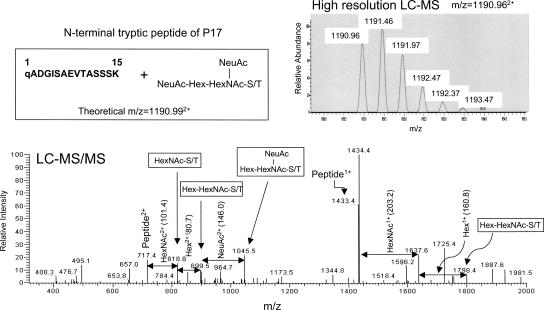
We then used the extracted ion current chromatogram (XIC) technique to confirm that the N-terminal tryptic peptide of P17 is modified with O-glycan {NeuAc(2)HexHexNAc}. Consequently, a doubly charged ion (+2) of the P17 peptide was observed at m/z 1190.962+ (Fig. 3, upper panel), which is almost identical to that calculated for the N-terminal tryptic peptide with an O-glycan {NeuAc(2)HexHexNAc} (m/= 1190.992+). Moreover, the LC-MS/MS analysis also supported the presence of a mucin-type O-glycan structure {NeuAc(2)HexHexNAc} in the N-terminal tryptic peptide of P17 (Fig. 3, lower panel).
Figure 3.
LC-MS and LC-MS/MS spectra of the N-terminal tryptic peptide of P17; “q” denotes pyro-Glu.
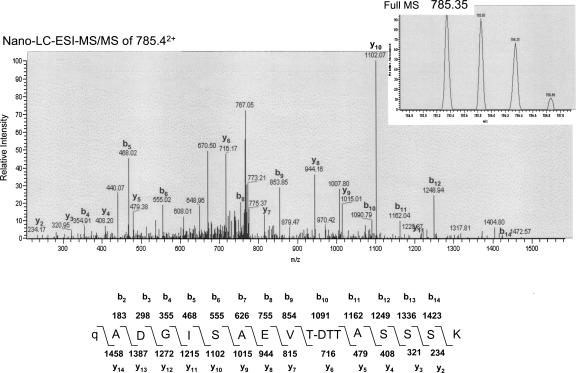
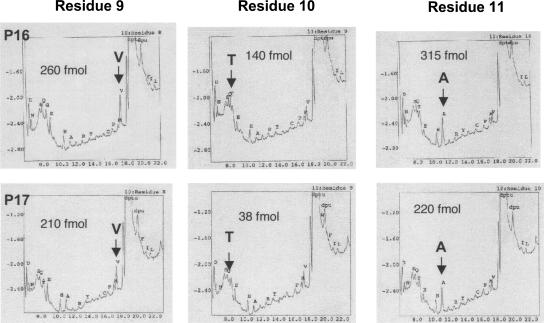
From the LC-MS/MS spectrum, we could not determine the site of O-glycosylation in the N-terminal tryptic peptide of P17 because little b- or y-type peptide ion was observed. However, the LC-MS/MS analysis of the peptide after treatment with dithiothreitol (DTT) under alkaline conditions (β-elimination followed by Michael addition with DTT, BEMAD) (Wells et al. 2002) detected a doubly charged ion derived from the P17 peptide at m/z = 785.352+ (Fig. 4, inset), which is almost identical to the MM calculated for the N-terminal tryptic peptide tagged with DTT at the O-glycosylated residue (m/z = 785.432+). Moreover, analysis of the b- or y-type peptide ions detected in the MS/MS spectrum identified that Thr10 is the O-glycosylation site in the peptide (Fig. 4). Furthermore, the N-terminal amino acid sequence analysis yielded an extremely low level of PTH–Thr from the 10th position of P17 compared with that from P16, while the yield of the neighboring PTH–amino acids was comparable for both proteins (Fig. 5). Therefore, we concluded that the P17 protein is O-glycosylated at Thr10.
Figure 4.
Determination of the O-glycosylation site in P17 by nano-LC-ESI-MS/MS analysis after BEMAD reaction. DTT replacement of O-linked oligosaccharides through BEMAD was performed on the tryptic peptides of P17. The calculated MM of the b and y peptide ions of the predicted structures are shown in the lower panel. Pyro-Glu and DTT-coupled Thr residues are denoted as “q” and “T-DTT.” Only prominent y and b fragment ions are labeled.
Figure 5.
Amino acid identification of positions 9, 10, and 11 in P16 and P17 by Edman degradation. Yield of PTH–amino acids is presented in each panel, indicating that the Thr residue (T) at position 10 of P17 is markedly lower than that of P16.
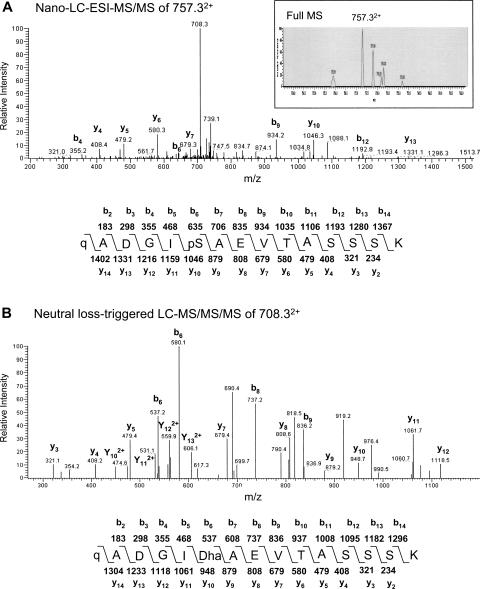
Identification of the phosphorylation site of P16 and P17
Analysis of the tryptic peptides of P16 using the LC-MS/MS neutral loss scanning method showed a doubly charged weak ion at m/z 757.32+ (Fig. 6A, inset) corresponding to the N-terminal tryptic peptide of P16 with a single phosphorylation (MM 1513.6 Da). Moreover, prominent b4, b6, b9, b12, y4, y5, y6, y7, y10, and y13 ions in the nano-LC-ESI-MS/MS spectrum all supported that serine at position 6 is phosphorylated (Fig. 6A). Further neutral loss-triggered LC-MS/MS/MS analysis of the m/z 708.32+ ion exhibited the series of y and b ions corresponding to the N-terminal tryptic peptide with a neutral loss of phosphoric acid from the Ser6 residue (Fig. 6B).
Figure 6.
Determination of the phosphorylation site in P16 by nano-LC-ESI-MS/MS and neutral loss-triggered LC-MS/MS/MS analyses. (A) Nano-LC-ESI-MS/MS spectrum of 757.33+ ion, detected by full MS analysis (inset). (B) Neutral loss-triggered LC-MS/MS/MS spectrum of 708.33+ ion that was detected by nano-LC-ESI-MS/MS spectrum presented in A. Calculated MM of b and y peptide ions of predicted structures are also shown. Phosphorylated Ser and pyro-Glu residues are denoted as “pS” and “q,” respectively. “Dha” indicates the site of the neutral loss of phosphoric acid from the phosphorylated Ser. Only prominent y and b fragment ions are labeled.
Collectively, post-translational modifications of rhBMP-15 are summarized in Figure 7. As shown, we demonstrate N-terminal pyroglutamic acid, phosphorylation at Ser6, O-glycosylation at Thr10, and C-terminal Lys truncation in rhBMP-15.
Figure 7.
Summary of post-translational modifications of rhBMP-15.
Discussion
N-Terminal pyro-Glu structure and C-terminal Lys truncation
In this article, we report that the N-terminal Gln of rhBMP-15 mature protein is cyclized to form pyro-Glu. It is known that N-terminal Gln and Glu are occasionally modified to pyro-Glu by Gln cyclase during protein synthesis. For example, the pro-proteins of gonadotropin-releasing hormone and thyrotropin-releasing hormone have Gln residues immediately following the dibasic cleavage sites of their respective precursor pro-proteins. Once these pro-proteins are cleaved, the N-terminal Gln of the mature proteins become pyro-Glu under physiological conditions (Richter et al. 1984; Seeburg and Adelman 1984; Lechan et al. 1986). While the biological relevance of the N-terminal pyro-Glu structure in rhBMP-15 is unknown, it may serve to confirm proper structure of the mature protein for its receptor binding and/or to protect it from degradation by extracellular aminopeptidases (Van Coillie et al. 1998; Hinke et al. 2000). Notably, the N-terminal amino acid of mature BMP-15 is highly conserved in many species (Fig. 8). Therefore, the pyro-Glu structure at the N terminus is likely to be a characteristic feature of the mammalian BMP-15 mature proteins.
Figure 8.
The N-terminal amino acid sequence alignment of the BMP-15 mature protein in different species.
One of the major limitations of the current immunohistochemical analysis of BMP-15 is that the presently available BMP-15 antibodies cannot distinguish the BMP-15 mature protein from the pro-protein in situ in the ovarian tissue sections. However, if the native BMP-15 mature protein has the N-terminal pyro-Glu structure after proteolytic cleavage of the pro-protein, we may be able to generate monoclonal antibodies that detect only the functional mature protein by immunohistochemistry for pathophysiological analysis. Moreover, monoclonal antibodies directed against the proteolytic cleavage site of the BMP-15 pro-protein could selectively detect BMP-15 pro-protein and not the mature protein. This approach may offer the advantage of direct comparison between the levels of BMP-15 mature and pro-proteins with respect to specific stages of folliculogenesis in situ (pre-antral, antral, dominant, and atretic follicles). These specific antibodies might facilitate in testing our hypothesis that the differences in the phenotypes caused by BMP-15 mutations between mono-ovulatory humans/sheep and polyovulatory mice may be attributed to the temporal variations in the production of the functional mature form of BMP-15 (Yoshino et al. 2006).
We also demonstrated that the C-terminal Lys of the Flag epitope that is fused to the C-terminus of rhBMP-15 is truncated. Truncation of the C-terminal Lys and Arg is not unusual in proteins produced by mammalian cell cultures (Harris 1995). Since the C-terminal Flag epitope has no effect on the biological activity of rhBMP-15, such a truncation is unlikely to alter the biological activity.
O-glycosylation
The role of O-linked oligosaccharides on the structure/function relationship in various proteins has been extensively studied. For example, the β-subunit of human chorionic gonadotropin (hCG) bears four O-linked oligosaccharide chains (Kessler et al. 1979), and they play important roles in the secretion of hCG from the cell, enhancement of bioactivity, and prolongation of its circulating half-life (Fares 2006). It is also known that defects in the synthesis of O-linked oligosaccharides and in the attachment of O-linked oligosaccharides to the backbone of the core proteins may result in some congenital diseases (Jaeken and Matthijs 2007). The physiological significance of O-glycosylation in BMP-15 is still unknown, as we have not yet succeeded in purifying sufficient amounts of the P16 and P17 proteins individually for their biological studies.
Phosphorylation
MALDI-TOF MS data indicate that a portion of the total P16 is phosphorylated at a single phosphorylation site. Further analysis by the LC-MS neutral loss method after in-gel digestion with trypsin revealed that Ser6 is phosphorylated. To our knowledge, BMP-15 is the only phosphorylated member of the TGF-β superfamily. Given that BMP-15 is a secreted protein, phosphorylation most likely takes place in the secretory pathways within the cells. In this regard, a casein kinase, termed Golgi apparatus casein kinase (G-CK), is a possible kinase for BMP-15 phosphorylation because (1) G-CK exerts its kinase activity in the Golgi apparatus (Lasa et al. 1997; Brunati et al. 2000; Tibaldi et al. 2006) and (2) studies with casein fractions and synthetic peptides as substrates for phosphorylation demonstrated that the consensus sequence targeted by G-CK is [Ser]-[Xaa]-[Glu or previously phosphorylated Ser] (Meggio et al. 1988, 1989; Lasa-Benito et al. 1996), which is overlapped with Ser6-Ala7-Glu8 in the human BMP-15 mature protein (see Fig. 8). This pattern of serine phosphorylation has been recognized not only in the milk caseins but also in many other proteins secreted into the extracellular environment, including adrenocorticotropin (Eipper and Mains 1982; Bennett et al. 1983), progastrin (Dockray et al. 1987; Varro et al. 1988; Desmond et al. 1989), interleukin 6 (May and Sehgal 1992), proenkephalin A (Watkinson et al. 1989), fibrinogen (Seydewitz et al. 1985), insulin-like growth factor binding protein-1 (Jones et al. 1993), matrix Gla protein (Price et al. 1994), and osteopontin (Sorensen et al. 1995; Lasa et al. 1997).
In conclusion, we have characterized the post-translational modification of rhBMP-15 mature protein (P16 and P17). Specifically, the N-terminal pyroglutamic acid structure and C-terminal Lys truncation are present in both the P16 and P17 forms. A phosphorylation at Ser6 in P16 and an O-glycosylation at Thr10 in P17 are also demonstrated. Post-translational modifications modulate the activity of most eukaryotic proteins, and their characterization provides indispensable insight into the biological function. Thus, identification of the modified amino acid residues in rhBMP-15 is an important step toward understanding the molecular basis of BMP-15 function. Although it is still unknown whether these post-translational modifications of rhBMP-15 are directly involved in the actions of BMP-15 in vivo, our findings may open a new area of investigation into these post-translational modifications in the regulation of BMP-15 bioactivity.
Materials and Methods
Materials and reagents
Ammonium bicarbonate, dithiothreitol (DTT), and sinapinic acid were purchased from Sigma. Modified sequence-grade trypsin was from Promega. Pfu pyroglutamate aminopeptidase was from TaKaRa Bio, Inc. Alkaline phosphatase (calf intestine) was from Roche, and triethylamine was from Fluka. Formic acid (>98%) was from Merck. SYPRO Ruby Protein Gel stain was obtained from Molecular Probes/Invitrogen. All other common reagents were purchased from Wako. rhBMP-15 tagged with a Flag epitope at the C terminus was produced by HEK293 cells and purified by using anti-Flag mAb as reported (Otsuka et al. 2000).
SDS-PAGE analysis
SDS-PAGE was performed following Laemmli's protocol (Laemmli 1970). Proteins were dissolved in SDS sample buffer (0.5 M Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 20 mM DTT, and 0.05% bromphenol blue), boiled for 5 min, and separated on a 12% acrylamide gel. The separated proteins were visualized by SYPRO Ruby staining according to the manufacturer's protocol or by Western immunoblotting using an anti-Flag mAb, as described previously (Otsuka et al. 2000).
Amino acid sequencing
Pfu pyroglutamate aminopeptidase digestion was performed to liberate the N-terminal pyro-Glu residue of the rhBMP-15 mature protein. Enzymatic digestion was carried out in 50 mM sodium phosphate (pH 7.0) containing 10 mM DTT and 1 mM EDTA at a ratio of 2 milliunits of enzyme to 1 nmol of the protein. The reaction mixture was incubated for 12 h at 55°C. Samples to be analyzed were subjected to 10% SDS-PAGE analysis, followed by electroblotting onto a polyvinylidine difluoride (PVDF) membrane. The bands on the PVDF membrane corresponding to P16 and P17 were excised and subjected to N-terminal amino acid sequencing via Edman degradation using a Procise 492 cLC sequencer (Applied Biosystems).
MALDI-TOF MS analysis
Samples were desalted and concentrated using Ultrafree 5K (Millipore) according to the manufacturer's instructions. The sinapinic acid was used as matrix by preparing a saturated solution of 33% (v/v) acetonitrile/0.1% (v/v) trifluoroacetic acid in water. Matrix (1 μL) and sample (1 μL) were thoroughly mixed together, and 0.5 μL of this mixture was spotted on the target plate and allowed to dry. MALDI-TOF MS analysis was performed on a Reflex III mass spectrometer (Bruker) equipped with pulsed ion extraction. All samples were irradiated with ultraviolet light (337 nm) from a N2 laser. Positive ions were accelerated to 20 kV and analyzed in a linear mode.
In-gel digestion and BEMAD
P17 and P16 protein bands stained with SYPRO Ruby were excised from the gel and subjected to trypsin digestion. Reductive alkylation and trypsin digestion were performed as previously described (Shevchenko et al. 1996). Briefly, SYPRO Ruby-stained bands were excised, dehydrated with acetonitrile, and reswollen in 25 mM ammonium bicarbonate. This was repeated twice, and the gel pieces were treated with 10 mM DTT for 1 h at 56°C and then with 55 mM iodoacetamide in the dark for 45 min at room temperature for carboxyamidomethylation. The gel was dehydrated and reswollen in 40 mM ammonium bicarbonate with 10 ng/μL modified sequence-grade trypsin on ice for 30 min. Excess trypsin was then washed off with 40 mM ammonium bicarbonate, followed by digestion overnight at 37°C. The tryptic peptides were extracted three times for 10 min each in 5% formic acid and 50% acetonitrile and then dehydrated using a Speed Vac. BEMAD was carried out according to the method described by Wells et al. (2002).
Liquid chromatography–mass (LC-MS) analysis
After drying, the peptide mixture was dissolved in 100 μL of 5% (v/v) acetonitrile-0.1% (v/v) formic acid and subjected to LC-electrospray ionization (ESI)-MS analysis. Specifically, a MAGIC2000 high-performance liquid chromatograph (MICHROM Bioresources, Inc.) equipped with a C18 column (C18 MonoCap Nano-flow, 0. 2 mm ID × 50 mm; GL Science) and an online-coupled linear-ion-trap mass spectrometer (LTQ-Orbitrap, Thermo Fisher Scientific Corp.) was used for this analysis. Elution was performed by applying a linear gradient of 5%–65% (v/v) acetonitrile in 0.1% (v/v) formic acid for 30 min. The data-dependent LC-MS/MS and neutral loss-triggered LC-MS/MS/MS fragmentation analyses were performed to determine tryptic peptides with a phosphorylated or O-glycosylated amino acid.
Peptide identification via MASCOT database search
Proteins were identified by automated database searching (Mascot Daemon, Matrix Science) against an in-house-created version of the BMP-15 protein sequence database. This database was complemented with frequently observed contaminants (porcine trypsin and human keratins). The following variable parameters were used for the search: an initial MS tolerance of ±10 ppm, a MS/MS tolerance of ±0.8 Da, and a full trypsin specificity allowing for up to two missed cleavages. In addition, the carbamidomethylated cysteine and oxidized methionine were chosen as a fixed and variable modification, respectively, in this search.
Acknowledgments
We thank Drs. Motoo Yamasaki and Michael Miller for valuable discussions and Mako Nakamura and Keiko Hiraishi for technical assistance. We also thank Andi Hartgrove for her assistance in preparing the manuscript. This work was supported in part by NIH Grant RO1 HD41494 and UCSD Academic Senate Grants RG008B and RH040H to S.S.
Footnotes
Supplemental material: see www.proteinscience.org
Reprint requests to: Shunichi Shimasaki, Department of Reproductive Medicine, UCSD, 9500 Gilman Drive, La Jolla, CA 92093-0633, USA; e-mail: sshimasaki@ucsd.edu; fax: (858) 822-1482.
Abbreviations: BMP-15, bone morphogenetic protein-15; ESI, electrospray ionization; GC, granulosa cell; G-CK, Golgi apparatus casein kinase; Hex, Hexose; LC, liquid chromatography; mAb, monoclonal antibody; MALDI-TOF, matrix-assisted laser-desorption/ionization time-of-flight; MS, mass spectrometry; MS/MS, tandem mass spectrometry; m/z, mass-to-charge ratio; NAc, N-acetyl group; NeuAc, N-acetylneuraminic acid; pyro-Glu, pyroglutamic acid; BEMAD, β-elimination followed by Michael addition with dithiothreitol.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.073232608.
References
- Bennett, H.P., Brubaker, P.L., Seger, M.A., Solomon, S. Human phosphoserine 31 corticotropin1-39. Isolation and characterization. J. Biol. Chem. 1983;258:8108–8112. [PubMed] [Google Scholar]
- Brunati, A.M., Contri, A., Muenchbach, M., James, P., Marin, O., Pinna, L.A. Grp94 (endoplasmin) co-purifies with and is phosphorylated by Golgi apparatus casein kinase. FEBS Lett. 2000;471:151–155. doi: 10.1016/s0014-5793(00)01378-8. [DOI] [PubMed] [Google Scholar]
- Carabatsos, M.J., Elvin, J., Matzuk, M.M., Albertini, D.F. Characterization of oocyte and follicle development in growth differentiation factor-9–deficient mice. Dev. Biol. 1998;204:373–384. doi: 10.1006/dbio.1998.9087. [DOI] [PubMed] [Google Scholar]
- Desmond, H., Varro, A., Young, J., Gregory, H., Nemeth, J., Dockray, G.J. The constitution and properties of phosphorylated and unphosphorylated C-terminal fragments of progastrin from dog and ferret antrum. Regul. Pept. 1989;25:223–233. doi: 10.1016/0167-0115(89)90264-4. [DOI] [PubMed] [Google Scholar]
- Dockray, G.J., Varro, A., Desmond, H., Young, J., Gregory, H., Gregory, R.A. Post-translational processing of the porcine gastrin precursor by phosphorylation of the COOH-terminal fragment. J. Biol. Chem. 1987;262:8643–8647. [PubMed] [Google Scholar]
- Dong, J., Albertini, D.F., Nishimori, K., Kumar, T.R., Lu, N., Matzuk, M. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- Dube, J.L., Wang, P., Elvin, J., Lyons, K.M., Celeste, A.J., Matzuk, M.M. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol. Endocrinol. 1998;12:1809–1817. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- Eipper, B.A., Mains, R.E. Phosphorylation of pro-adrenocorticotropin/endorphin-derived peptides. J. Biol. Chem. 1982;257:4907–4915. [PubMed] [Google Scholar]
- Elvin, J.A., Yan, C., Matzuk, M.M. Oocyte-expressed TGF-β superfamily members in female fertility. Mol. Cell. Endocrinol. 2000;159:1–5. doi: 10.1016/s0303-7207(99)00185-9. [DOI] [PubMed] [Google Scholar]
- Eppig, J.J. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- Erickson, G.F., Shimasaki, S. The role of the oocyte in folliculogenesis. Trends Endocrinol. Metab. 2000;11:193–198. doi: 10.1016/s1043-2760(00)00249-6. [DOI] [PubMed] [Google Scholar]
- Fares, F. The role of O-linked and N-linked oligosaccharides on the structure–function of glycoprotein hormones: Development of agonists and antagonists. Biochim. Biophys. Acta. 2006;1760:560–567. doi: 10.1016/j.bbagen.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Gregory, K.E., Ono, R.N., Charbonneau, N.L., Kuo, C.L., Keene, D.R., Bachinger, H.P., Sakai, L.Y. The prodomain of BMP-7 targets the BMP-7 complex to the extracellular matrix. J. Biol. Chem. 2005;280:27970–27980. doi: 10.1074/jbc.M504270200. [DOI] [PubMed] [Google Scholar]
- Harris, R.J. Processing of C-terminal lysine and arginine residues of proteins isolated from mammalian cell culture. J. Chromatogr. A. 1995;705:129–134. doi: 10.1016/0021-9673(94)01255-d. [DOI] [PubMed] [Google Scholar]
- Hayashi, M., McGee, E.A., Min, G., Klein, C., Rose, U.M., Van Duin, M., Hsueh, A.J.W. Recombinant growth differentiation factor-9 (GDF-9) enhances growth and differentiation of cultured early ovarian follicles. Endocrinology. 1999;140:1236–1244. doi: 10.1210/endo.140.3.6548. [DOI] [PubMed] [Google Scholar]
- Hinke, S.A., Pospisilik, J.A., Demuth, H.U., Mannhart, S., Kuhn-Wache, K., Hoffmann, T., Nishimura, E., Pederson, R.A., McIntosh, C.H. Dipeptidyl peptidase IV (DPIV/CD26) degradation of glucagon. Characterization of glucagon degradation products and DPIV-resistant analogs. J. Biol. Chem. 2000;275:3827–3834. doi: 10.1074/jbc.275.6.3827. [DOI] [PubMed] [Google Scholar]
- Jaatinen, R., Laitinen, M., Vuojolainen, K., Aaltonen, J., Louhio, H., Heikinheimo, K., Lehtonen, E., Ritvos, O. Localization of growth differentiation factor-9 (GDF-9) mRNA and protein in rat ovaries and cDNA cloning of rat GDF-9 and its novel homolog GDF-9b. Mol. Cell. Endocrinol. 1999;156:189–193. doi: 10.1016/s0303-7207(99)00100-8. [DOI] [PubMed] [Google Scholar]
- Jaeken, J., Matthijs, G. Congenital disorders of glycosylation: A rapidly expanding disease family. Annu. Rev. Genomics Hum. Genet. 2007;8:261–278. doi: 10.1146/annurev.genom.8.080706.092327. [DOI] [PubMed] [Google Scholar]
- Jiang, M.S., Liang, L.F., Wang, S., Ratovitski, T., Holmstrom, J., Barker, C., Stotish, R. Characterization and identification of the inhibitory domain of GDF-8 propeptide. Biochem. Biophys. Res. Commun. 2004;315:525–531. doi: 10.1016/j.bbrc.2004.01.085. [DOI] [PubMed] [Google Scholar]
- Jones, J.I., Busby W.H., Jr, Wright, G., Smith, C.E., Kimack, N.M., Clemmons, D.R. Identification of the sites of phosphorylation in insulin-like growth factor binding protein-1. Regulation of its affinity by phosphorylation of serine 101. J. Biol. Chem. 1993;268:1125–1131. [PubMed] [Google Scholar]
- Julenius, K., Mølgaard, A., Gupta, R., Brunak, S. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology. 2005;15:153–164. doi: 10.1093/glycob/cwh151. [DOI] [PubMed] [Google Scholar]
- Kessler, M.J., Mise, T., Ghai, R.D., Bahl, O.P. Structure and location of the O-glycosidic carbohydrate units of human chorionic gonadotropin. J. Biol. Chem. 1979;254:7909–7914. [PubMed] [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laitinen, M., Vuojolainen, K., Jaatinen, R., Ketola, I., Aaltonen, J., Lehtonen, E., Heikinheimo, M., Ritvos, O. A novel growth differentiation factor-9 (GDF-9) related factor is co-expressed with GDF-9 in mouse oocytes during folliculogenesis. Mech. Dev. 1998;78:135–140. doi: 10.1016/s0925-4773(98)00161-0. [DOI] [PubMed] [Google Scholar]
- Lasa, M., Chang, P.L., Prince, C.W., Pinna, L.A. Phosphorylation of osteopontin by Golgi apparatus casein kinase. Biochem. Biophys. Res. Commun. 1997;240:602–605. doi: 10.1006/bbrc.1997.7702. [DOI] [PubMed] [Google Scholar]
- Lasa-Benito, M., Marin, O., Meggio, F., Pinna, L.A. Golgi apparatus mammary gland casein kinase: Monitoring by a specific peptide substrate and definition of specificity determinants. FEBS Lett. 1996;382:149–152. doi: 10.1016/0014-5793(96)00136-6. [DOI] [PubMed] [Google Scholar]
- Lechan, R.M., Wu, P., Jackson, I.M., Wolf, H., Cooperman, S., Mandel, G., Goodman, R.H. Thyrotropin-releasing hormone precursor: Characterization in rat brain. Science. 1986;231:159–161. doi: 10.1126/science.3079917. [DOI] [PubMed] [Google Scholar]
- Lee, S.J., McPherron, A.C. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, W.X., Moore, R.K., Otsuka, F., Shimasaki, S. Effect of intracellular interactions on the processing and secretion of bone morphogenetic protein-15 (BMP-15) and growth and differentiation factor-9: Implication of the aberrant ovarian phenotype of BMP-15 mutant sheep. J. Biol. Chem. 2003;278:3713–3719. doi: 10.1074/jbc.M210598200. [DOI] [PubMed] [Google Scholar]
- May, L.T., Sehgal, P.B. Phosphorylation of interleukin-6 at serine-54: An early event in the secretory pathway in human fibroblasts. Biochem. Biophys. Res. Commun. 1992;185:524–530. doi: 10.1016/0006-291x(92)91656-b. [DOI] [PubMed] [Google Scholar]
- McGrath, S.A., Esquela, A.F., Lee, S.-J. Oocyte-specific expression of growth/differentiation factor-9. Mol. Endocrinol. 1995;9:131–136. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- McPherron, A.C., Lee, S.-J. GDF-3 and GDF-9: Two new members of the transforming growth factor-β superfamily containing a novel pattern of cysteines. J. Biol. Chem. 1993;268:3444–3449. [PubMed] [Google Scholar]
- Meggio, F., Boulton, A.P., Marchiori, F., Borin, G., Lennon, D.P., Calderan, A., Pinna, L.A. Substrate-specificity determinants for a membrane-bound casein kinase of lactating mammary gland. A study with synthetic peptides. Eur. J. Biochem. 1988;177:281–284. doi: 10.1111/j.1432-1033.1988.tb14374.x. [DOI] [PubMed] [Google Scholar]
- Meggio, F., Perich, J.W., Meyer, H.E., Hoffmann-Posorske, E., Lennon, D.P., Johns, R.B., Pinna, L.A. Synthetic fragments of β-casein as model substrates for liver and mammary gland casein kinases. Eur. J. Biochem. 1989;186:459–464. doi: 10.1111/j.1432-1033.1989.tb15229.x. [DOI] [PubMed] [Google Scholar]
- Otsuka, F., Shimasaki, S. A negative feedback system between oocyte bone morphogenetic protein 15 and granulosa cell kit ligand: Its role in regulating granulosa cell mitosis. Proc. Natl. Acad. Sci. 2002;99:8060–8065. doi: 10.1073/pnas.122066899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka, F., Yamamoto, S., Erickson, G.F., Shimasaki, S. Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J. Biol. Chem. 2001;276:11387–11392. doi: 10.1074/jbc.M010043200. [DOI] [PubMed] [Google Scholar]
- Otsuka, F., Yao, Z., Lee, T.H., Yamamoto, S., Erickson, G.F., Shimasaki, S. Bone morphogenetic protein-15: Identification of target cells and biological functions. J. Biol. Chem. 2000;275:39523–39528. doi: 10.1074/jbc.M007428200. [DOI] [PubMed] [Google Scholar]
- Price, P.A., Rice, J.S., Williamson, M.K. Conserved phosphorylation of serines in the Ser-X-Glu/Ser(P) sequences of the vitamin K-dependent matrix Gla protein from shark, lamb, rat, cow, and human. Protein Sci. 1994;3:822–830. doi: 10.1002/pro.5560030511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, K., Kawashima, E., Egger, R., Kreil, G. Biosynthesis of thyrotropin releasing hormone in the skin of Xenopus laevis: Partial sequence of the precursor deduced from cloned cDNA. EMBO J. 1984;3:617–621. doi: 10.1002/j.1460-2075.1984.tb01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg, P.H., Adelman, J.P. Characterization of cDNA for precursor of human luteinizing hormone releasing hormone. Nature. 1984;311:666–668. doi: 10.1038/311666a0. [DOI] [PubMed] [Google Scholar]
- Seydewitz, H.H., Kaiser, C., Witt, I. The location of a second in vivo phosphorylation site in the Aa chain of human fibrinogen. In: Henschen A., editor. Fibrinogen—structural variants and interactions. Walter de Gruyter & Co; Berlin, Germany: 1985. pp. 121–132. [Google Scholar]
- Shevchenko, A., Wilm, M., Vorm, O., Mann, M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Shimasaki, S., Moore, R.K., Otsuka, F., Erickson, G.F. The bone morphogenetic protein system in mammalian reproduction. Endocr. Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- Sorensen, E.S., Hojrup, P., Petersen, T.E. Post-translational modifications of bovine osteopontin: Identification of twenty-eight phosphorylation and three O-glycosylation sites. Protein Sci. 1995;4:2040–2049. doi: 10.1002/pro.5560041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies, R.S., Chen, T.J., Davies, M.V., Tomkinson, K.N., Pearson, A.A., Shakey, Q.A., Wolfman, N.M. GDF-8 propeptide binds to GDF-8 and antagonizes biological activity by inhibiting GDF-8 receptor binding. Growth Factors. 2000;18:251–259. doi: 10.3109/08977190109029114. [DOI] [PubMed] [Google Scholar]
- Tibaldi, E., Arrigoni, G., Brunati, A.M., James, P., Pinna, L.A. Analysis of a sub-proteome which co-purifies with and is phosphorylated by the Golgi casein kinase. Cell. Mol. Life Sci. 2006;63:378–389. doi: 10.1007/s00018-005-5506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Coillie, E., Proost, P., Van Aelst, I., Struyf, S., Polfliet, M., De Meester, I., Harvey, D.J., Van Damme, J., Opdenakker, G. Functional comparison of two human monocyte chemotactic protein-2 isoforms, role of the amino-terminal pyroglutamic acid and processing by CD26/dipeptidyl peptidase IV. Biochemistry. 1998;37:12672–12680. doi: 10.1021/bi980497d. [DOI] [PubMed] [Google Scholar]
- Varro, A., Desmond, H., Pauwels, S., Gregory, H., Young, J., Dockray, G.J. The human gastrin precursor. Characterization of phosphorylated forms and fragments. Biochem. J. 1988;256:951–957. doi: 10.1042/bj2560951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson, A., Young, J., Varro, A., Dockray, G.J. The isolation and chemical characterization of phosphorylated enkephalin-containing peptides from bovine adrenal medulla. J. Biol. Chem. 1989;264:3061–3065. [PubMed] [Google Scholar]
- Wells, L., Vosseller, K., Cole, R.N., Cronshaw, J.M., Matunis, M.J., Hart, G.W. Mapping sites of O-GlcNAC modification using affinity tags for serine and threonine post-translational modifications. Mol. Cell. Proteomics. 2002;1:791–804. doi: 10.1074/mcp.m200048-mcp200. [DOI] [PubMed] [Google Scholar]
- Yoshino, O., McMahon, H.E., Sharma, S., Shimasaki, S. A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. Proc. Natl. Acad. Sci. 2006;103:10678–10683. doi: 10.1073/pnas.0600507103. [DOI] [PMC free article] [PubMed] [Google Scholar]