Abstract
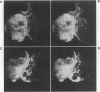
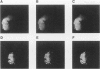
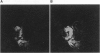
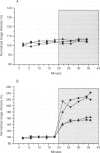
Gradient-recalled echo magnetic resonance imaging (GRE MRI), which gives information on blood flow and oxygenation changes (Robinson SP, Howe FA, Griffiths JR 1995, Int J Radiat Oncol Biol Phys 33: 855), was used to observe the responses of six rodent tumour models to carbogen breathing. In one transplanted rat tumour, the Morris hepatoma 9618a, and a chemically induced rat tumour, the MNU-induced mammary adenocarcinoma, there were marked image intensity increases, similar to those previously observed in the rat GH3 prolactinoma. In contrast, the rat Walker carcinosarcoma showed no response. In two mouse tumours, the RIF-1 fibrosarcoma and the human xenograft HT29, carbogen breathing induced a transient fall in signal intensity that reversed spontaneously within a few minutes. The rat GH3 prolactinoma was xenografted into nude mice, and an increase in image intensity was found in response to carbogen, suggesting that any effects that carbogen may have had on the host were not significant determinants of the tumour response. The increases in GRE image intensity of the MNU, H9618a and GH3 tumours during carbogen breathing are consistent with increases in tumour oxygenation and blood flow, whereas the responses of the RIF-1 and HT29 tumours may be the result of a transient steal effect followed by homeostatic correction.
Full text
PDF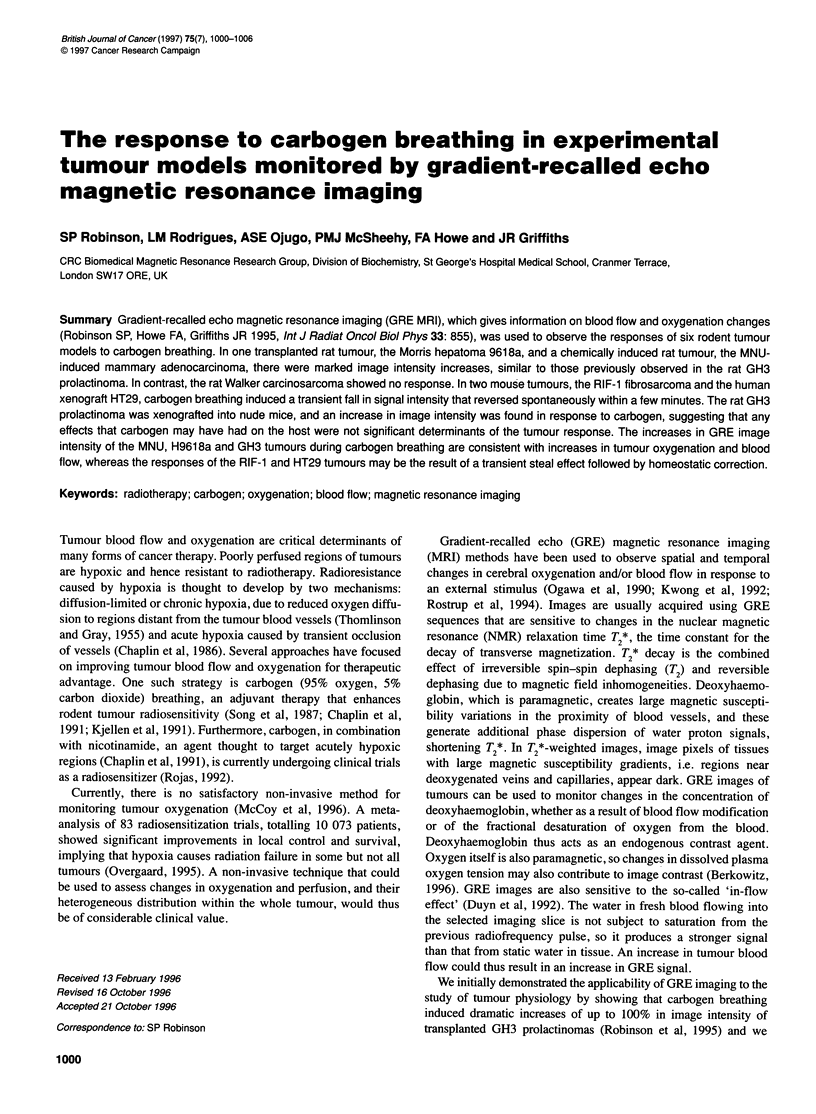
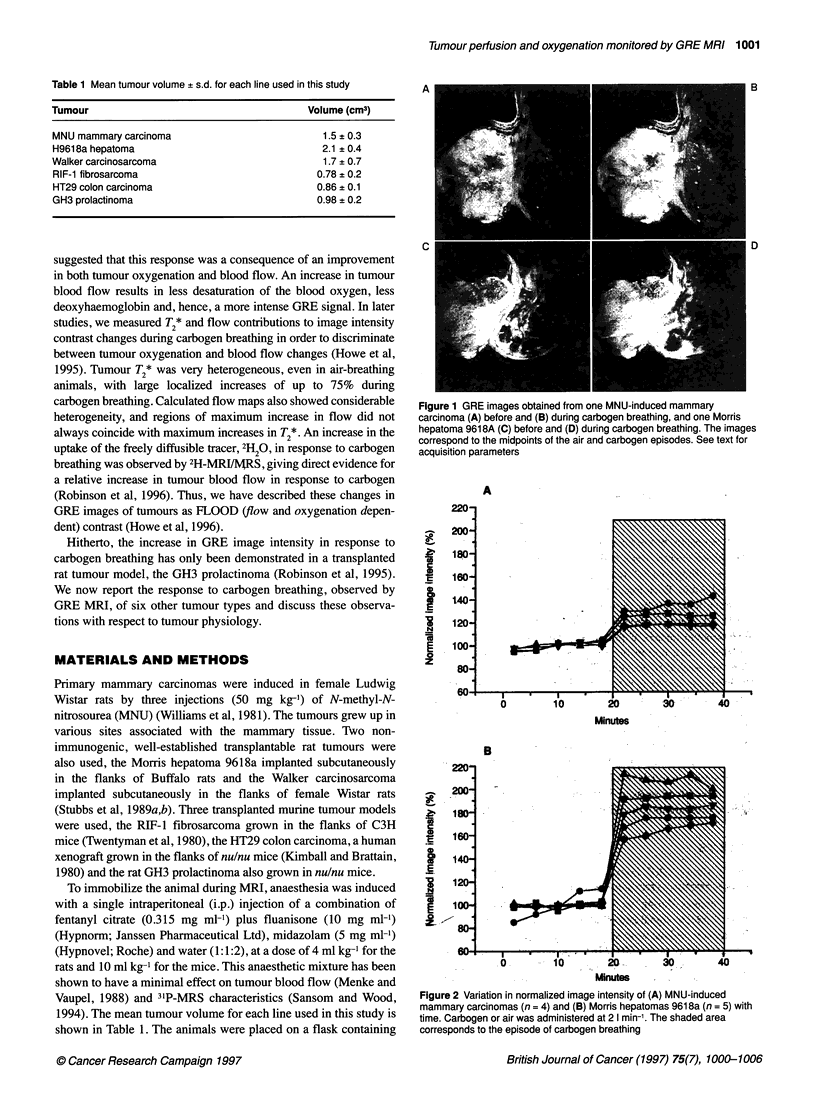
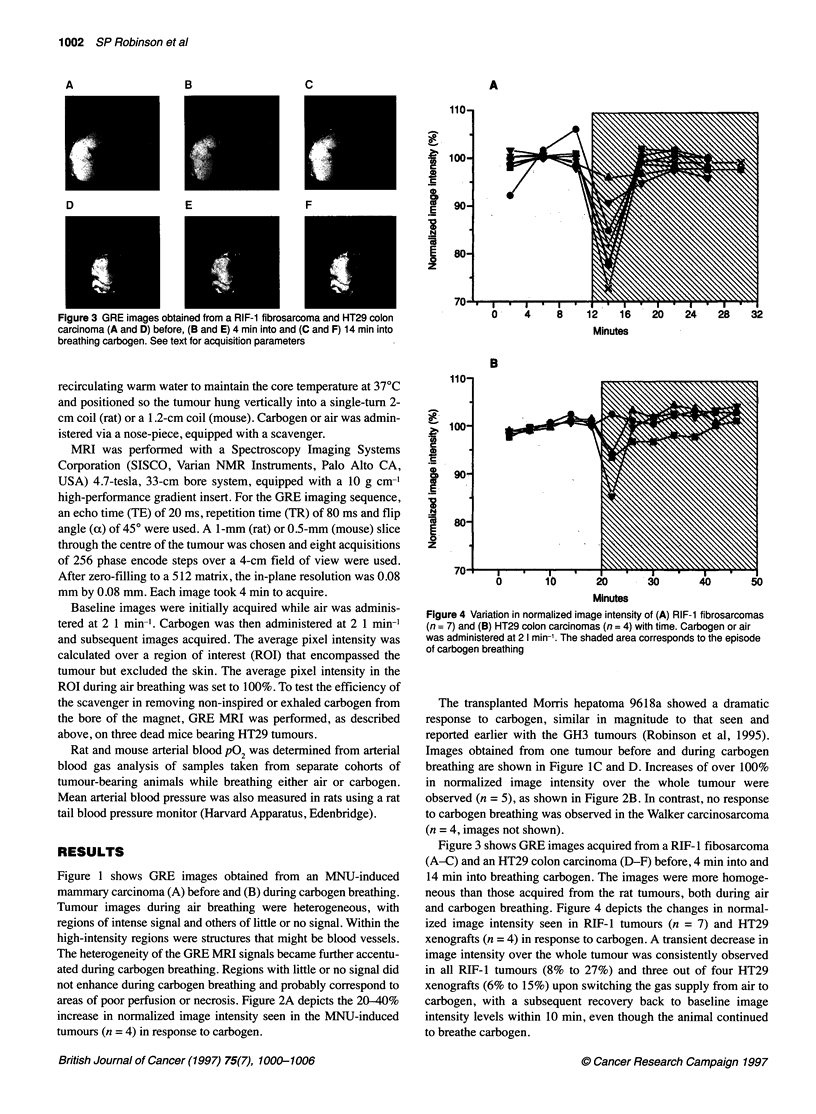
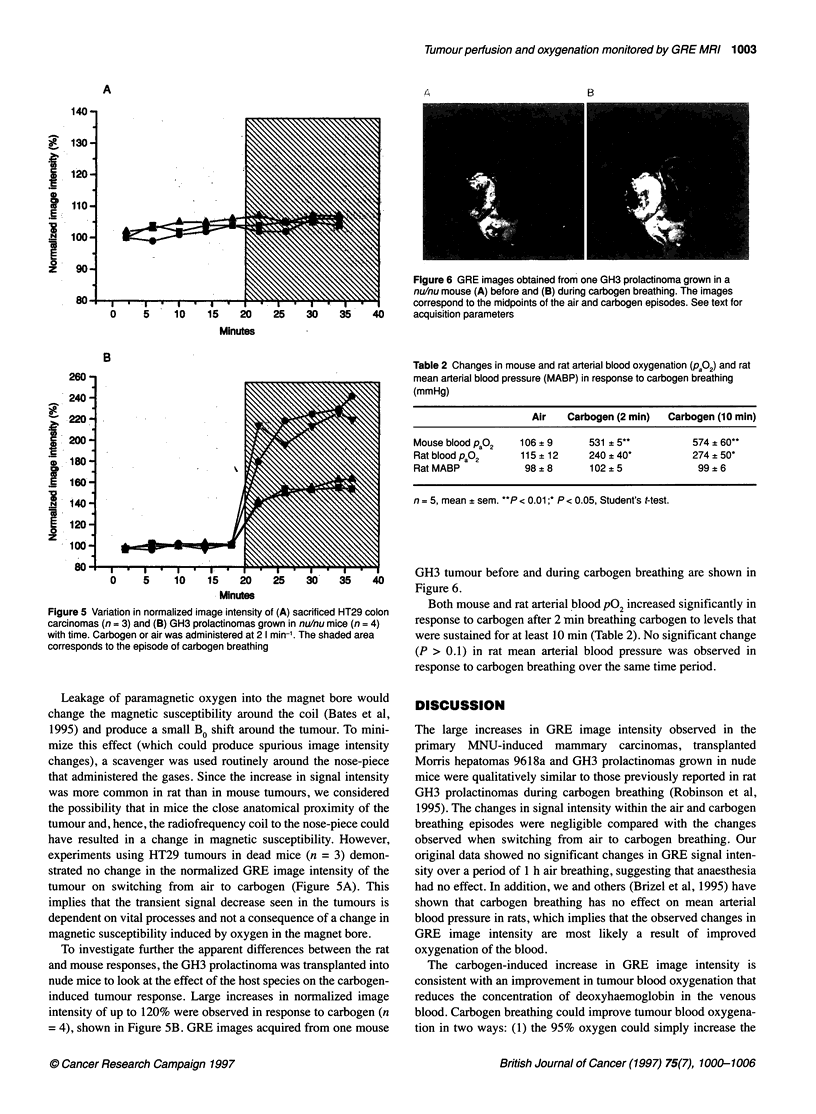



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates S., Yetkin Z., Jesmanowicz A., Hyde J. S., Bandettini P. A., Estkowski L., Haughton V. M. Artifacts in functional magnetic resonance imaging from gaseous oxygen. J Magn Reson Imaging. 1995 Jul-Aug;5(4):443–445. doi: 10.1002/jmri.1880050413. [DOI] [PubMed] [Google Scholar]
- Calais G., Hirst D. G. In situ tumour radiosensitization induced by clofibrate administration: single dose and fractionated studies. Radiother Oncol. 1991 Oct;22(2):99–103. doi: 10.1016/0167-8140(91)90004-z. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Durand R. E., Olive P. L. Acute hypoxia in tumors: implications for modifiers of radiation effects. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1279–1282. doi: 10.1016/0360-3016(86)90153-7. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Horsman M. R., Aoki D. S. Nicotinamide, Fluosol DA and Carbogen: a strategy to reoxygenate acutely and chronically hypoxic cells in vivo. Br J Cancer. 1991 Jan;63(1):109–113. doi: 10.1038/bjc.1991.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyn J. H., Moonen C. T., van Yperen G. H., de Boer R. W., Luyten P. R. Inflow versus deoxyhemoglobin effects in BOLD functional MRI using gradient echoes at 1.5 T. NMR Biomed. 1994 Mar;7(1-2):83–88. doi: 10.1002/nbm.1940070113. [DOI] [PubMed] [Google Scholar]
- Falk P. Differences in vascular pattern between the spontaneous and the transplanted C3H mouse mammary carcinoma. Eur J Cancer Clin Oncol. 1982 Feb;18(2):155–165. doi: 10.1016/0277-5379(82)90059-1. [DOI] [PubMed] [Google Scholar]
- Falk S. J., Ward R., Bleehen N. M. The influence of carbogen breathing on tumour tissue oxygenation in man evaluated by computerised p02 histography. Br J Cancer. 1992 Nov;66(5):919–924. doi: 10.1038/bjc.1992.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY L. H., STEADMAN J. M. DETERMINATION OF THE OXYHAEMOGLOBIN DISSOCIATION CURVES FOR MOUSE AND RAT BLOOD. J Physiol. 1964 Dec;175:161–171. doi: 10.1113/jphysiol.1964.sp007509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess D. J., Bleehen N. M. Perfusion changes in the RIF-1 tumour and normal tissues after carbogen and nicotinamide, individually and combined. Br J Cancer. 1995 Jun;71(6):1175–1180. doi: 10.1038/bjc.1995.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball P. M., Brattain M. G. Isolation of a cellular subpopulation from a human colonic carcinoma cell line. Cancer Res. 1980 May;40(5):1574–1579. [PubMed] [Google Scholar]
- Kjellen E., Joiner M. C., Collier J. M., Johns H., Rojas A. A therapeutic benefit from combining normobaric carbogen or oxygen with nicotinamide in fractionated X-ray treatments. Radiother Oncol. 1991 Oct;22(2):81–91. doi: 10.1016/0167-8140(91)90002-x. [DOI] [PubMed] [Google Scholar]
- Kwong K. K., Belliveau J. W., Chesler D. A., Goldberg I. E., Weisskoff R. M., Poncelet B. P., Kennedy D. N., Hoppel B. E., Cohen M. S., Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence V. M., Ward R., Dennis I. F., Bleehen N. M. Carbogen breathing with nicotinamide improves the oxygen status of tumours in patients. Br J Cancer. 1995 Jul;72(1):198–205. doi: 10.1038/bjc.1995.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy C. L., McIntyre D. J., Robinson S. P., Aboagye E. O., Griffiths J. R. Magnetic resonance spectroscopy and imaging methods for measuring tumour and tissue oxygenation. Br J Cancer Suppl. 1996 Jul;27:S226–S231. [PMC free article] [PubMed] [Google Scholar]
- Menke H., Vaupel P. Effect of injectable or inhalational anesthetics and of neuroleptic, neuroleptanalgesic, and sedative agents on tumor blood flow. Radiat Res. 1988 Apr;114(1):64–76. [PubMed] [Google Scholar]
- Ogawa S., Lee T. M., Nayak A. S., Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990 Apr;14(1):68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Howe F. A., Griffiths J. R. Noninvasive monitoring of carbogen-induced changes in tumor blood flow and oxygenation by functional magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 1995 Nov 1;33(4):855–859. doi: 10.1016/0360-3016(95)00072-1. [DOI] [PubMed] [Google Scholar]
- Rojas A. ARCON: accelerated radiotherapy with carbogen and nicotinamide. BJR Suppl. 1992;24:174–178. [PubMed] [Google Scholar]
- Rostrup E., Larsson H. B., Toft P. B., Garde K., Thomsen C., Ring P., Søndergaard L., Henriksen O. Functional MRI of CO2 induced increase in cerebral perfusion. NMR Biomed. 1994 Mar;7(1-2):29–34. doi: 10.1002/nbm.1940070106. [DOI] [PubMed] [Google Scholar]
- Sansom J. M., Wood P. J. 31P MRS of tumour metabolism in anaesthetized vs conscious mice. NMR Biomed. 1994 Jun;7(4):167–171. doi: 10.1002/nbm.1940070403. [DOI] [PubMed] [Google Scholar]
- Song C. W., Lee I., Hasegawa T., Rhee J. G., Levitt S. H. Increase in pO2 and radiosensitivity of tumors by Fluosol-DA (20%) and carbogen. Cancer Res. 1987 Jan 15;47(2):442–446. [PubMed] [Google Scholar]
- Stubbs M., Rodrigues L. M., Griffiths J. R. Growth studies of subcutaneous rat tumours: comparison of 31P-NMR spectroscopy, acid extracts and histology. Br J Cancer. 1989 Nov;60(5):701–707. doi: 10.1038/bjc.1989.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs M., Rodrigues L. M., Griffiths J. R. Potential artefacts from overlying tissues in 31P NMR spectra of subcutaneously implanted rat tumours. NMR Biomed. 1989 Apr;1(4):165–170. doi: 10.1002/nbm.1940010403. [DOI] [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twentyman P. R., Brown J. M., Gray J. W., Franko A. J., Scoles M. A., Kallman R. F. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980 Mar;64(3):595–604. [PubMed] [Google Scholar]
- Williams J. C., Gusterson B., Humphreys J., Monaghan P., Coombes R. C., Rudland P., Neville A. M. N-methyl-N-nitrosourea-induced rat mammary tumors. Hormone responsiveness but lack of spontaneous metastasis. J Natl Cancer Inst. 1981 Jan;66(1):147–155. [PubMed] [Google Scholar]
- Wood P. J., Stratford I. J., Sansom J. M., Cattanach B. M., Quinney R. M., Adams G. E. The response of spontaneous and transplantable murine tumors to vasoactive agents measured by 31P magnetic resonance spectroscopy. Int J Radiat Oncol Biol Phys. 1992;22(3):473–476. doi: 10.1016/0360-3016(92)90856-d. [DOI] [PubMed] [Google Scholar]