Abstract
The role of the lytic enzyme beta-(1,3)-glucanase in cell wall synthesis and its distribution in the mycelium of the fungus Sclerotium rolfsii were studied. Enzyme activity was determined after enzyme extraction with Triton X-100 from a cell wall preparation. Specific zones of immunofluorescence appeared in the hyphal tips, clamp connections, new septa, and lateral branching when a specific antiserum was used with the indirect method of the fluorescent antibody staining. Enzymatic activity in the cell wall preparation was inactivated by diethylpyrocarbonate. However, 69% of the total enzymatic activity was present in a latent form which was not affected by the ester. This result suggests that most of the beta-(1,3)-glucanase was present along the hyphal cell walls in a "masked" form. An active enzyme appeared only in those regions which showed immunofluorescence. The activity of glucan synthetase, an enzyme essential for wall formation, was higher in the branching funus grown on L-threonine-supplemented synthetic medium than in the synthetic medium-grown fungus.
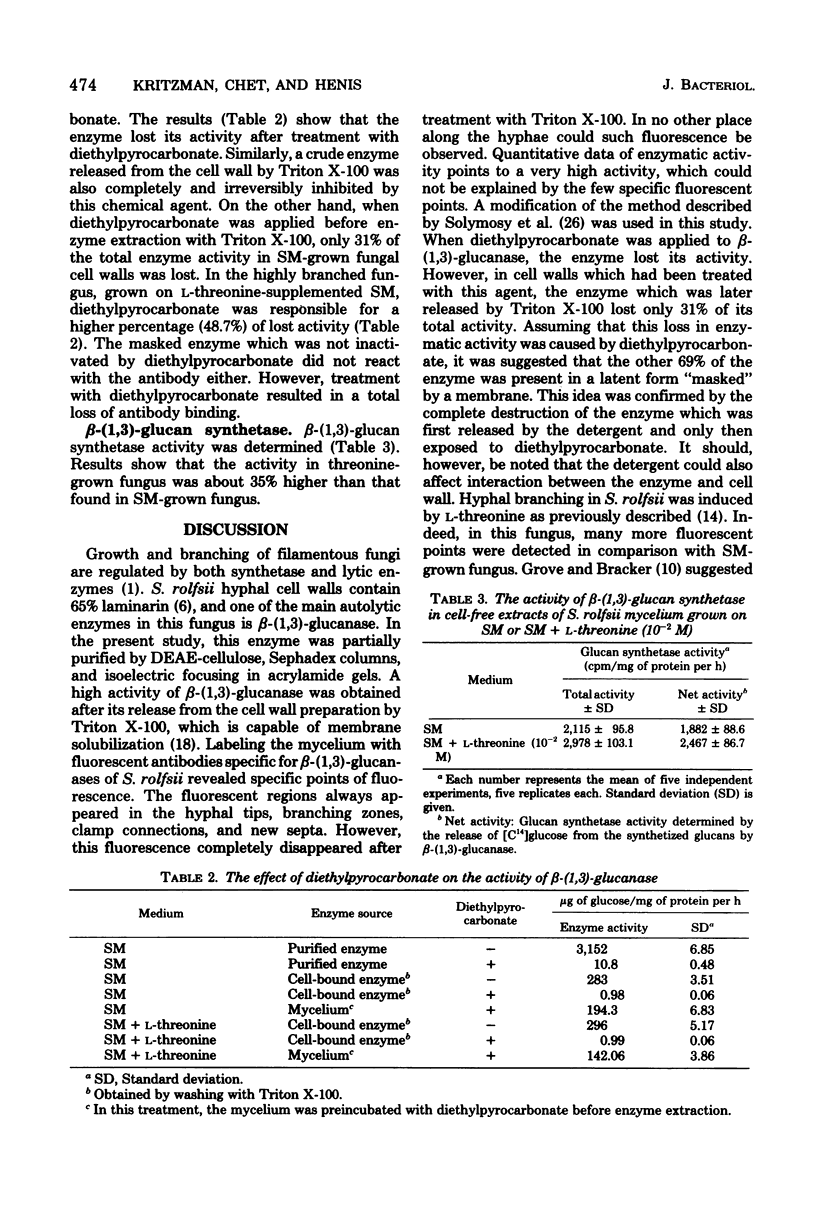
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chet I., Henis Y., Mitchell R. Chemical composition of hyphal and sclerotial walls of Sclerotium rolfsii Sacc. Can J Microbiol. 1967 Feb;13(2):137–141. doi: 10.1139/m67-019. [DOI] [PubMed] [Google Scholar]
- Comp P. C., Lester G. Properties of an extracellular -galactosidase secreted by Neurospora crassa. J Bacteriol. 1971 Jul;107(1):162–167. doi: 10.1128/jb.107.1.162-167.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe B., Holldorf A. W. Control of Extracellular beta-1,3-glucanase activity in a basidiomycete species. J Bacteriol. 1975 Jun;122(3):818–825. doi: 10.1128/jb.122.3.818-825.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg E. Lactic and Malic Dehydrogenases in Human Spermatozoa. Science. 1963 Feb 15;139(3555):602–603. doi: 10.1126/science.139.3555.602. [DOI] [PubMed] [Google Scholar]
- Grove S. N., Bracker C. E. Protoplasmic organization of hyphal tips among fungi: vesicles and Spitzenkörper. J Bacteriol. 1970 Nov;104(2):989–1009. doi: 10.1128/jb.104.2.989-1009.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Jones D., Gordon A. H., Bacon J. S. Co-operative action by endo- and exo-beta-(1 leads to 3)-glucanases from parasitic fungi in the degradation of cell-wall glucans of Sclerotinia sclerotiorum (Lib.) de Bary. Biochem J. 1974 Apr;140(1):47–55. doi: 10.1042/bj1400047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzman G., Chet I., Henis Y. The relationship between rhythmic hyphal growth and circadian formation of sclerotia in Sclerotium rolfsii Sacc. Can J Microbiol. 1977 Aug;23(8):959–963. doi: 10.1139/m77-143. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ne'eman Z., Kahane I., Razin S. Characterization of the mycoplasma membrane proteins. II. Solubilization and enzymic activities of Acholeplasma laidlawii membrane proteins. Biochim Biophys Acta. 1971 Oct 12;249(1):169–176. doi: 10.1016/0005-2736(71)90093-9. [DOI] [PubMed] [Google Scholar]
- REESE E. T., MANDELS M. Beta-D-1, 3 Glucanases in fungi. Can J Microbiol. 1959 Apr;5(2):173–185. doi: 10.1139/m59-022. [DOI] [PubMed] [Google Scholar]
- Solymosy F., Fedorcsák I., Gulyás A., Farkas G. L., Ehrenberg L. A new method based on the use of diethyl pyrocarbonate as a nuclease inhibitor for the extraction of undegraded nucleic acid from plant tissues. Eur J Biochem. 1968 Sep 24;5(4):520–527. doi: 10.1111/j.1432-1033.1968.tb00401.x. [DOI] [PubMed] [Google Scholar]





