Abstract
We have investigated the function of the synaptic vesicle protein Rabphilin-3A in neurotransmitter release at the squid giant synapse. Presynaptic microinjection of recombinant Rabphilin-3A reversibly inhibited the exocytotic release of neurotransmitter. Injection of fragments of Rabphilin-3A indicate that at least two distinct regions of the protein inhibit neurotransmitter release: the NH2-terminal region that binds Rab3A and is phosphorylated by protein kinases and the two C2 domains that interact with calcium, phospholipid, and β-adducin. Each of the inhibitory fragments and the full-length protein had separate effects on presynaptic morphology, suggesting that individual domains were inhibiting a subset of the reactions in which the full-length protein participates. In addition to inhibiting exocytosis, constructs containing the NH2 terminus of Rabphilin-3A also perturbed the endocytotic pathway, as indicated by changes in the membrane areas of endosomes, coated vesicles, and the plasma membrane. These results indicate that Rabphilin-3A regulates synaptic vesicle traffic and appears to do so at distinct stages of both the exocytotic and endocytotic pathways.
Keywords: endocytosis, exocytosis, GTP-binding proteins, rabs, neurotransmitter release
introduction
Synaptic vesicles undergo many reactions within presynaptic nerve terminals. Vesicles accumulate at active zones, the presynaptic sites of neurotransmitter release, and a subset dock via attachment to the plasma membrane (Couteaux and Pecot-Dechavassine, 1974; Schweizer et al., 1995). In response to calcium influx during a presynaptic action potential, docked synaptic vesicles fuse with the plasma membrane to release neurotransmitter into the synaptic cleft (Katz, 1966; Heuser et al., 1979). After fusion, the vesicle components are endocytosed, probably in a dynamin- and clathrin-dependent manner, to yield coated vesicles (DeCamilli, 1995). These coated vesicles lose their coats and coalesce with early endosomes (Heuser and Reese, 1973; DeHoop et al., 1994; but see Takei et al., 1996; Koenig and Ikeda, 1996). New vesicles are thought to bud from these endosomes, refill with neurotransmitter and reenter the pool of vesicles available for docking (Heuser and Reese, 1973; DeCamilli, 1995). The molecular mechanisms that mediate the transitions through this cycle of exocytosis and endocytosis are largely unknown (Kelly, 1993a ; Scheller, 1995; Südhof, 1995; Augustine et al., 1996).
One potential member of the vesicular trafficking machinery is the synaptic vesicle–associated protein Rabphilin-3A. Rabphilin-3A was first identified as a binding partner of Rab3A (Shirataki et al., 1993, 1994), a member of the Rab family of GTPases implicated in vesicle docking/fusion reactions in many systems (Simons and Zerial, 1993; Takai et al., 1996). Rabphilin-3A binds to the GTP-bound form of Rab3A (Yamaguchi et al., 1993) and maintains Rab3A in this state by preventing a GTPase-activating protein from catalyzing GTP hydrolysis by Rab3A (Kishida et al., 1993). The Rab3A binding site is localized to amino acid residues 40–170 and includes a conserved stretch of cysteines (Stahl et al., 1996). The NH2-terminal half of Rabphilin-3A also binds to the cytoskeletal protein, α-actinin, and enhances its ability to bundle actin filaments (Kato et al., 1996). The COOH terminus of Rabphilin-3A contains two C2 domains that bind calcium and phospholipid and are homologous to the C2 domains of synaptotagmin, a protein suggested to be the calcium sensor that triggers vesicle fusion (DeBello et al., 1993; Südhof, 1995). In addition, the C2 domains of Rabphilin-3A bind to the cytoskeletal protein β-adducin (Miyazaki et al., 1994), which functions in the assembly of spectrin–actin complexes at the plasma membrane (Miche et al., 1987; Hughes and Bennett, 1995). Although Rabphilin-3A is associated with synaptic vesicles (Shirataki et al., 1994; Li et al., 1994), the protein lacks a membrane-spanning region. Instead, Rabphilin-3A is thought to associate with synaptic vesicles by binding to another vesicular protein whose identity is not yet clear (Shirataki et al., 1994; McKiernan et al., 1996; Stahl et al., 1996).
The biochemical properties of Rabphilin-3A, in particular its ability to bind calcium, Rab3A, and the cytoskeleton, make it a prospective participant in synaptic vesicle trafficking (Südhof, 1997). We have examined the function of this protein in the synaptic vesicle cycle by microinjecting Rabphilin-3A or protein fragments of Rabphilin-3A into nerve terminals of the giant synapse of the squid. We have found that Rabphilin-3A and two of its fragments potently inhibit neurotransmitter release. Further, these reagents differentially affect endocytotic compartments. We interpret these results to indicate that Rabphilin-3A regulates both exo- and endocytosis.
materials and methods
Electrophysiology
Stellate ganglia of the squid, Loligo pealei, were removed from 10– 20-cm-long animals and perfused with a saline (466 mM NaCl, 54 mM MgCl2, 11 mM CaCl2, 10 mM KCl, 3 mM NaHCO3, 10 mM HEPES, pH 7.2) oxygenated with a 99.5%-O2/0.5%-CO2 mixture. During experiments, this solution was cooled to 13–15°C with a Peltier device (Cambion, Cambridge, MA). All experiments were performed on an upright Optiphot microscope with a 10× objective (Nikon Inc., Melville, NY). A microelectrode containing 3 M KCl was placed into the presynaptic axon to deliver current pulses (400–1,000 nA; 0.033 Hz) to elicit action potentials. A second electrode containing 3 M KCl was inserted into the postsynaptic cell to record postsynaptic potential (PSPs)1 resulting from neurotransmitter release in response to a presynaptic action potential. To detect large changes in the rate of spontaneous miniature PSPs, the postsynaptic membrane potential was monitored at high gain throughout the course of the experiment and recorded on a chart recorder. A third electrode, used both for microinjection of proteins and for recording the presynaptic action potential, was inserted directly into the presynaptic terminal of the most distal giant synapse. This injection electrode contained the proteins or protein fragments in an internal solution designed to mimic squid axoplasm while maintaining proper protein folding (250 mM NaCl, 50 mM KCl, 50 mM taurine, 125 mM K-isethionate, 0.5 mM DTT, 35 mM HEPES, pH 7.4).
To estimate the volume of solution injected, 100 μM fluorescein dextran (10 kD) was included in the injection electrode. An Odyssey confocal microscope (Noran Instruments, Middleton, WI) was used to measure the fluorescence intensity of the terminal throughout the course of the experiment. To determine the concentration of dextran injected, this fluorescence signal was compared with a standard calibration curve constructed from known concentrations of fluorescein dextran in microcuvettes approximating the path length of the terminal (50 μm; Martin and Miledi, 1986). This estimate of the intracellular concentration of fluorescein dextran was then converted to protein concentration from the following relationship: [protein]in = ([F-dextran]in × [protein]pip)/[F-dextran]pip, where [protein]in = intracellular concentration of protein; [F-dextran]in = intracellular concentration of fluorescein dextran; [protein]pip = concentration of protein in injection electrode; [F-dextran]pip = concentration of fluorescein dextran in injection electrode.
Protein Expression and Purification
Recombinant bovine full-length Rabphilin-3A, the HA-tagged NH2-terminal fragment (residues 1–280), and the HA-tagged COOH-terminal fragment (residues 396–704) were purified from the membrane fraction of overexpressing Sf 9 insect cells (Shirataki et al., 1994; Kikuchi et al., 1995). The glutathione-S-transferase (GST)–COOH-terminal fragment (residues 281– 704) was purified from overexpressing Escherichia coli (Shirataki and Takai, 1995), and the GST carrier was cleaved off from the COOH-terminal fragment by digestion with thrombin.
Ultrastructural Analysis
Electron microscopy.
Single presynaptic terminals microinjected with various reagents were examined with electron microscopy. Tissue was fixed and processed as described by Sanchez et al. (1990). Briefly, after microinjection of a Rabphilin-3A protein that abolished transmitter release or a comparable volume of a control solution (buffer alone, HA tag, or inactive Rabphilin-3A fragment), the electrodes were rapidly removed and the entire ganglion was immersed in fixative containing 2% glutaraldehyde, 800 mM sucrose, and 3% DMSO in 100 mM Na-cacodylate buffer. After standard histological processing, sections were cut from each terminal at 50-μm intervals. Each 80-nm-thick section was magnified 12,000 times using an electron microscope (JEM-1200ExII; Jeol U.S.A. Inc., Peabody, MA), and every active zone encountered was photographed and analyzed. This paradigm yielded 10 or more sections per terminal and resulted in images of ∼200 active zones per terminal.
For each experimental or control condition, at least two terminals were used and the active zones of the two finally pooled into one group. Whenever possible, the same batch of fixative solution was used to preserve both control and experimental terminals to eliminate any sources of variability related to fixation conditions. Photographs of active zones were digitized and analyzed using commercial software (Image-1; Universal Imaging Corp., West Chester, PA).
Vesicle spatial distributions.
Spatial distributions of vesicles were determined by measuring the distance between the plasma membrane and the center of each synaptic vesicle within 0.5 μm of the plasma membrane at a given active zone. This was performed for all active zones in all sections of each terminal. These distances were binned into 50-nm compartments to yield the average number of synaptic vesicles located in a particular bin at an active zone. To further compare these distributions independent of differences in the total vesicle number, the number of vesicles in each compartment also have been expressed as a percentage of the total vesicles at a given active zone.
Surface area measurements.
For each section, the surface area of the entire plasma membrane of the presynaptic terminal, including synaptic and nonsynaptic regions, was determined at low magnification by measuring the perimeter of the terminal and multiplying by the section thickness. All other organelles found within 1.5 μm of the plasma membrane at an active zone were measured at high magnification as follows: the diameters of coated vesicles and synaptic vesicles were measured; since these organelles are spherical and thinner than a section, their areas were calculated as A = 4π r2. The mean diameters of these vesicles were measured and did not change as a consequence of injecting any Rabphilin-3A reagent. The surface area of large organelles was determined by measuring the perimeter and multiplying by section thickness. Some endosome-like structures were irregularly shaped and others were circular in profile. We believe these structures represent two distinct classes of endosomes (Miller and Heuser, 1984; Kelly, 1993b ; Mundigl et al., 1993; DeHoop et al., 1994) and have therefore categorized their membrane areas separately.
Five active zones in each section were randomly selected for surface area measurements. The total area of membranes in each membrane compartment for each section was divided by five to determine the mean amount of membrane per active zone. This average value was then multiplied by the total number of active zones present in each section to determine the extrapolated amount of total membrane per section. This extrapolation was necessary for meaningful comparisons of the area of the synaptic membrane compartments to that of the surface area of the plasma membrane, which includes sites of endocytosis that lie outside the immediate region of the active zone. Values from 20 sections (from two terminals) were used to calculate the group values shown in Fig. 4. Grouping the data by terminals instead of by sections (n = 2 instead of 20) yielded the same results and frequently displayed even less variance.
Figure 4.
Rabphilin-3A redistributes membranes among organelles of the synaptic vesicle cycle. The surface area of several organelles was measured in 20 sections taken from terminals injected with either full-length Rabphilin-3A (FL), the 1–280 fragment (N), the 396–704 fragment (C), or control solutions (Con). Although these treatments produced significant changes (*P < 0.05) in the membrane area of individual organelles, the total amount of organellar membrane (bottom right) was conserved.
results
Rabphilin-3A Inhibits Neurotransmitter Release
To determine the function of Rabphilin-3A in neurotransmitter release, we began by microinjecting recombinant full-length bovine Rabphilin-3A into the squid giant presynaptic terminal. Microinjection of Rabphilin-3A into the presynaptic terminal resulted in a reduction in the postsynaptic potential evoked by presynaptic action potentials (n = 14; Fig. 1 A). Because Rabphilin-3A was injected only into the presynaptic cell, this inhibition of the PSP is due to a reduction in neurotransmitter release. Rabphilin-3A injection produced no change in presynaptic resting or action potentials, indicating that inhibition of neurotransmitter release is not due to an indirect effect on presynaptic ion channels. Likewise, there was no change in the waveform of the PSP that would indicate a desynchronization of neurotransmitter release (Schweizer et al., 1998). Since the rate of rise of the PSP (d V/dt) is proportional to the amount of transmitter released by the presynaptic cell (Augustine et al., 1985), this measure was used to monitor the time course of action of Rabphilin-3A (Fig. 1 B, top). Inhibition by Rabphilin-3A proceeded rapidly, beginning <1 min after starting the injection. Further, this inhibition was a specific effect of injecting Rabphilin-3A; neurotransmitter release was not affected by even larger injections of protein-free buffer or another C2 domain–containing protein, Doc-2 (not shown).
Figure 1.
Rabphilin-3A inhibits neurotransmitter release. Microinjection of Rabphilin-3A reversibly inhibited the postsynaptic potential PSP evoked by presynaptic action potentials (A). The time course of Rabphilin-3A inhibition of PSP slope (d V/dt), normalized to the maximum value measured during the experiment (B), was correlated with the amount of protein that was microinjected. Microinjection of Rabphilin-3A during the time indicated by solid horizontal bars (top) was monitored by coinjection of 10 kD fluorescein dextran, which was used to estimate the concentration of injected protein (bottom; see methods). The extent of inhibition was a function of the intracellular concentration of Rabphilin-3A, measured at the time of peak inhibition and at 10 min before and after (C). These data were fit with the Hill equation; half-maximal inhibition required ∼500 nM Rabphilin-3A. The data points are the mean ± SEM of one to three injections to each concentration.
Rabphilin-3A was capable of inhibiting transmission completely if the amount of Rabphilin-3A injected into the terminal was sufficient. To quantify the amount of Rabphilin-3A required to inhibit neurotransmitter release, fluorescein-conjugated dextran was used as a volume marker and injected along with Rabphilin-3A. The brightness of the dextran within the terminal was measured to estimate the concentration of Rabphilin-3A injected into the terminal (Fig. 1 B). The amount of neurotransmitter released declined (top) as the amount of Rabphilin-3A within the terminal increased (bottom). Because the rate of diffusion of dextran and protein within the terminal are likely to be different, we estimated the amount of Rabphilin-3A injected only at short times (<10 min) after the onset of injection, when any differences in the relative concentrations of the two should be minimal. The relationship between the peak concentration of Rabphilin-3A injected and the amount of inhibition that resulted was determined from several separate injection experiments (Fig. 1 C). This relationship can be described by the Hill equation, with half-maximal inhibition requiring ∼0.5 μM Rabphilin-3A.
The inhibition of neurotransmitter release resulting from Rabphilin-3A injection was reversible. Release began to recover shortly after terminating the injection, and proceeded in a manner correlated with the decline in fluorescence intensity (Fig. 1 B, bottom). This reversibility is likely to be due to the injected protein diffusing out of the terminal and into the rest of the large presynaptic neuron, causing a reduction in Rabphilin-3A concentration within the terminal. The full reversibility of inhibition shows that Rabphilin-3A does not bind irreversibly to its presynaptic target(s). It also provides another indication that the inhibition of transmitter release observed with Rabphilin-3A injection is not a result of mechanical damage to the presynaptic terminal, because such damage is not commonly reversible.
Rabphilin-3A Regulates Exo- and Endocytosis
To determine the site within the synaptic vesicle cycle at which Rabphilin-3A acts to inhibit neurotransmitter release, we used electron microscopy to examine the morphological consequences of injecting this protein. Two terminals were injected with sufficient Rabphilin-3A to abolish PSPs, and then were rapidly fixed. Control terminals were injected with comparable volumes of buffer alone, and then processed identically. Active zones from control terminals or those injected with Rabphilin-3A both contained an abundance of synaptic vesicles (Fig. 2). To quantify the spatial distribution of synaptic vesicles, we have measured the distance between the plasma membrane and the center of each of these synaptic vesicles (Hess et al., 1993; Bommert et al., 1993; Hunt et al., 1994; DeBello et al., 1995) at hundreds of active zones from each set of terminals. Neither the raw distributions (Fig. 3 A, left) nor the distributions normalized to the number of total vesicles (Fig. 3 A, right) revealed changes in the spatial distribution of synaptic vesicles within 0.5 μm of the plasma membrane at the active zones, despite the full inhibition of neurotransmitter release by Rabphilin-3A.
Figure 2.

Rabphilin-3A injection alters presynaptic structure. Representative images of active zones from terminals injected with either buffer alone or the HA tag (Control), full-length Rabphilin-3A (Rabphilin-3A), the 1–280 (NH2-terminal) fragment, or the 396–704 (COOH-terminal) fragment. CV, coated vesicle; ISE, irregularly-shaped endosome; VE, vesicular endosome.
Figure 3.
Differential actions of Rabphilin-3A fragments on the spatial distribution of synaptic vesicles. The mean number of synaptic vesicles (SVs) found in 50-nm spatial compartments within the active zone (AZ) was determined for presynaptic terminals injected with control solutions or Rabphilin-3A proteins (left). These data are also expressed as a percentage of the total number of vesicles at a given active zone (right). Comparison of terminals injected with buffer alone (n = 482 active zones) or full-length Rabphilin-3A (n = 321 active zones) revealed no change in the spatial distribution of synaptic vesicles despite full inhibition of transmitter release (A). The spatial distribution of vesicles at active zones from terminals injected with the 1–280 fragment (B; n = 423 active zones) is significantly different from that of controls injected with buffer alone or an inactive NH2-terminal fragment (n = 300 active zones). Likewise, the 396–704 fragment (C; n = 381 active zones) also differed from controls injected with the HA tag (n = 356 active zones). Both fragments reduced vesicle number in each spatial compartment (left) and caused a relative accumulation of vesicles 50–100 nm from the plasma membrane (right).
To determine whether Rabphilin-3A affects other organelles of the synaptic vesicle cycle, we next examined endocytotic as well as exocytotic membrane compartments in terminals injected with Rabphilin-3A. Quantitation of presynaptic membrane cycling usually involves measuring the surface areas of four membrane-bound compartments involved in this cycle (Heuser and Reese, 1973): synaptic vesicles (SVs), coated vesicles (CVs), endosomes, and plasma membrane. Since the size and shape of endosomes change under conditions that affect endocytotic vesicle traffic (Heuser and Reese, 1973; Bucci et al., 1992; DeHoop et al., 1994; Takei et al., 1996), we have expanded this categorization by subdividing endosomes into two types: irregularly shaped endosomes (ISE) and circular structures larger than synaptic vesicles (typically 80–200 nm diameter; vesicular endosomes, VE). We measured the surface area of the entire plasma membrane and the surface area of the other four categories of organelles (SV, CV, ISE, VE) located within 1.5 μm of the plasma membrane of the active zone.
In comparison with controls, terminals injected with Rabphilin-3A displayed no change in the area of synaptic vesicle membrane (Fig. 4), confirming that Rabphilin-3A inhibited exocytosis without affecting the synaptic vesicle population. In addition, the protein had no effect on the area of the irregularly shaped endosomal compartments. However, terminals injected with Rabphilin-3A showed a 2.5-fold increase in the membrane area of vesicular endosomes; this was a significant increase over the area of these organelles in control terminals (P < 10−5 by t test). This increase in both the size and number of vesicular endosomes was accompanied by a decrease in membrane within coated vesicles (P < 0.001) and the plasma membrane (P < 0.01). The total amount of membrane was constant between terminals injected with Rabphilin-3A and control solutions (Fig. 4, bottom right), supporting the conclusion that Rabphilin-3A caused a redistribution of membrane by promoting the formation of vesicular endosomal structures from coated vesicles and the plasma membrane.
Transmitter release results from the fusion of docked vesicles (Heuser et al., 1979; Torri-Tarelli et al., 1985), and terminals inhibited by Rabphilin-3A possessed a full complement of synaptic vesicles at the active zone. Thus, while our structural analysis indicates that injection of Rabphilin-3A can promote the formation of endosomal intermediates, it does not identify the structural basis for the decrease in neurotransmitter release caused by injecting this protein. A plausible hypothesis is that this protein also participates in reactions that make docked synaptic vesicles capable of undergoing fusion (see discussion).
The Rab3-binding Domain of Rabphilin-3A also Regulates Vesicle Traffic
Rabphilin-3A consists of several domains with distinct binding properties. Recombinant fragments of Rabphilin-3A were injected into the presynaptic terminal to identify the roles of these domains in regulating neurotransmitter release (Fig. 5 A). These fragments should bind to a subset of Rabphilin-3A targets and thus inhibit the function of endogenous Rabphilin-3A by competing for its binding partners inside the cell.
Figure 5.

The NH2-terminal fragment of Rabphilin-3A inhibits transmitter release. Fragments encompassing the various domains of Rabphilin-3A were selected for microinjection (A). Microinjection of the 1–280 fragment inhibited neurotransmitter release in a manner correlated with the time and extent of injection (B). The degree of inhibition was a saturating function of the 1–280 fragment, with half-maximal inhibition occurring at 0.8 μM (C). The data points are the mean ± SEM of one to seven injections to each concentration.
The binding site for Rab3A is within residues 70–140 of the NH2 terminus of Rabphilin-3A (Stahl et al., 1996). A recombinant fragment of Rabphilin-3A encompassing this site (residues 1–280; Fig. 5 A) binds to Rab3A and inhibits Rab3A-GTPase-activating protein in a manner similar to the full-length protein (Yamaguchi et al., 1993; Kishida et al., 1993). This 1–280 fragment was also a potent inhibitor of transmitter release when microinjected into the squid presynaptic terminal (Fig. 5 B; n = 19). The inhibitory effect was not due to the HA tag of the protein construct because injection of the HA tag alone had no effect on neurotransmitter release, even at 500–1,000-fold higher concentrations (not shown). The inhibition of release produced by the 1–280 fragment was reversible and dose dependent, with half-maximal inhibition requiring ∼0.8 μM of this fragment (Fig. 5 D). Thus, in regard to its specificity, reversibility, and dose dependence, the 1–280 fragment inhibited transmitter release in a manner similar to that of the full-length protein. This suggests that at least some of the biological activity of Rabphilin-3A resides within this domain.
To identify the site of action of the 1–280 fragment in the synaptic vesicle cycle, we examined the morphology of terminals injected with this fragment. These terminals exhibited marked morphological changes in comparison to controls (Fig. 2). Analysis of the spatial distribution of synaptic vesicles revealed two consequences of injecting the 1–280 fragment. First, this fragment reduced the total number of synaptic vesicles within 0.5 μm of the plasma membrane, evident as a decrease in the number of vesicles in each spatial compartment (Fig. 3 B, left). However, this fragment did not reduce vesicle number uniformly; instead, the fragment changed the shape of the distribution, causing a selective and significant (P ≅ 0) relative accumulation (or reduced amount of depletion) of synaptic vesicles located 50–100 nm from the plasma membrane (Fig. 3 B, right). Because the vesicles responsible for transmitter release must be located in close proximity to the plasma membrane, it seems likely that the observed changes in these vesicles are related to the inhibition of neurotransmitter release produced by this fragment (see discussion).
The NH2-terminal fragment could reduce the number of vesicles by stimulating spontaneous exocytosis, disrupting vesicle recruitment to the active zone, or by blocking an endocytotic pathway. To test whether the 1–280 fragment increased spontaneous fusion events, the frequency of spontaneous miniature PSPs (Miledi, 1967; Mann and Joyner, 1982; Augustine and Eckert, 1984) was estimated by monitoring the resting potential of the postsynaptic cell at high gain (Miledi, 1973; Charlton et al., 1982; Augustine et al., 1988). However, injection of neither this fragment nor any other Rabphilin-3A derivative produced any change in the postsynaptic resting potential (data not shown), although injection of depolarizing current into the presynaptic terminal to increase the rate of spontaneous release did elicit small (1–3-mV) postsynaptic depolarizations (Charlton and Atwood, 1977; Augustine and Eckert, 1984). Thus, the NH2-terminal fragment apparently does not reduce vesicle number by stimulating the rate of spontaneous exocytosis.
To determine whether the NH2-terminal fragment reduced vesicle number by blocking endocytosis, we measured organelle surface areas in terminals injected with the NH2-terminal fragment (Fig. 4). This fragment reduced the area of synaptic vesicle membrane (P < 0.01), increased the surface area of the plasma membrane (P < 10−5), and reduced the membrane area (and number) of coated vesicles (P < 0.05). Because the total amount of organelle membrane did not differ significantly between groups, (Fig. 4, bottom right), these results suggest that the membrane lost from the synaptic vesicle pool was relocated to the plasma membrane. Thus, in contrast to full-length Rabphilin-3A, which apparently promoted endocytosis, the 1–280 fragment appeared to inhibit endocytotic membrane retrieval from the plasma membrane.
C2 Domains of Rabphilin-3A also Affect Neurotransmitter Release and Membrane Traffic
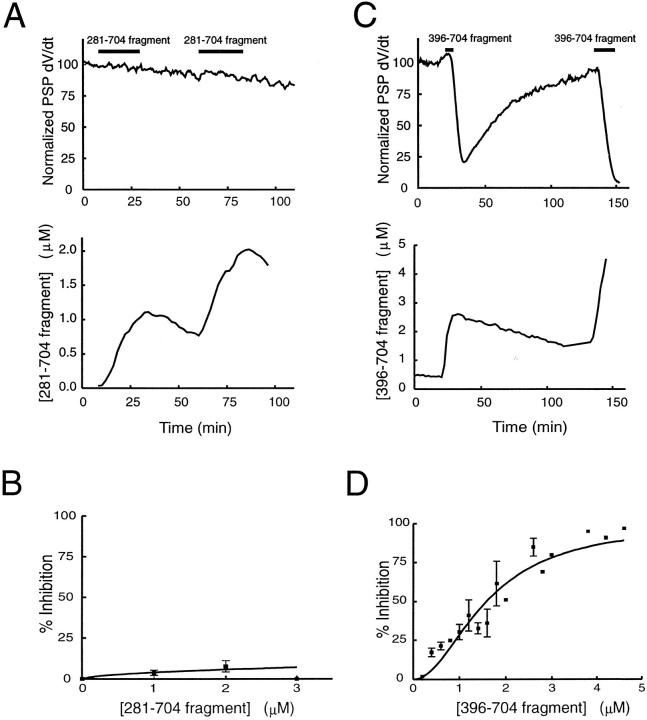
To determine whether the COOH-terminal domain of Rabphilin-3A also plays a role in regulating neurotransmitter release, two COOH-terminal fragments were selected for microinjection (Fig. 5 A). One fragment contained the entire COOH-terminal half of Rabphilin (amino acid residues 281–704), while the second contained only the C2 domains (residues 396–704). Both of these fragments bind to calcium, lipid, and β-adducin (Yamaguchi et al., 1993; Miyazaki et al., 1994). Injection of the 281–704 fragment had no effect on neurotransmitter release (Fig. 6 A, n = 14), even when high concentrations of this fragment were injected (Fig. 6 B). In contrast, injection of the shorter 396–704 fragment inhibited transmitter release reversibly (Fig. 6 C; n = 16). The effect of this fragment was dose dependent, requiring 1.7 μM protein for half-maximal inhibition (Fig. 6 D). These results indicate that the COOH-terminal region also contributes to the biological activity of Rabphilin-3A.
Figure 6.
The C2 domains of Rabphilin-3A regulate transmitter release. Microinjection of the 281–704 fragment of Rabphilin-3A has no effect on neurotransmitter release (A), even at concentrations of several micromolar (B; one to five injections to each concentration). However, microinjection of the 396–704 fragment did inhibit release reversibly (C). This inhibition was dose dependent (D ; one to seven injections to each concentration), with half-maximal inhibition requiring injection of ∼1.7 μM of this fragment.
Like the 1–280 fragment, the 396–704 fragment also altered the morphology of the presynaptic terminal (Fig. 2). Compared with control terminals injected with the HA tag, injection of the COOH-terminal fragment reduced the number of vesicles in all spatial compartments within 0.5 μm of the plasma membrane (Fig. 3 C, left) and, like the 1–280 fragment, caused a relative accumulation (P < 10−8) of vesicles located 100 nm from the plasma membrane (Fig. 3 C, right). These similarities suggest that the 396–704 fragment might inhibit exocytosis via the same mechanisms as the 1–280 fragment. However, unlike the 1–280 fragment, the 396–704 fragment did not appear to inhibit endocytosis because surface area measurements indicated no statistically significant change in any membrane-bound compartment of terminals injected with this fragment (Fig. 4). Thus, while the COOH-terminal domain inhibits exocytosis, it does not affect the endocytotic pathway.
The COOH-terminal fragment decreased the number of synaptic vesicles within 0.5 μm of the plasma membrane (14.3 ± 0.5 per active zone compared with 24.7 ± 0.8 in controls; n = 100 active zones; P < 10−50; Fig. 3 C), yet the surface area measurements (which included synaptic vesicles up to 1.5 μm from the plasma membrane) showed no significant difference in synaptic vesicle area. Therefore, we wondered if the COOH-terminal fragment may have prevented the translocation of vesicles close to the plasma membrane. To assess this possibility, we measured the number of vesicles located at greater distances from the plasma membrane (0.5–1.0 μm). The COOH-terminal fragment did not significantly decrease the number of vesicles far (0.5–1.0 μm) from the plasma membrane (34.1 ± 3.9 per active zone, compared with 41.8 ± 2.9 in controls; n = 100 active zones; P > 0.1). The mechanism by which this fragment reduced vesicle number remains to be determined; it is possible that it either disperses the vesicle cluster close to the active zone or prevents vesicles far from the membrane from translocating to the release sites.
discussion
We have found that bovine Rabphilin-3A and its fragments potently inhibit neurotransmitter release when microinjected into the presynaptic terminal of squid. Indeed, Rabphilin-3A is the most active reagent among the >50 that we have injected into the squid giant presynaptic terminal (Burns et al., 1996). Although squid Rabphilin-3A has not yet been identified, squid presynaptic proteins typically are highly homologous to their mammalian counterparts (Bommert et al., 1993; Hunt et al., 1994; DeBello et al., 1995; Burns et al., 1996; O'Connor et al., 1997; Dresbach et al., 1998). Thus, there is strong reason to suspect that Rabphilin-3A is present in squid presynaptic terminals and has a primary sequence homologous to the mammalian protein that we have injected.
Neurotransmitter release requires the functional coupling of exocytosis and endocytosis of synaptic vesicle membrane (Heuser and Reese, 1973), so that the inhibition of transmitter release that we observed after Rabphilin-3A injection could have been the result of perturbing any stage of the synaptic vesicle trafficking cycle. By combining electrophysiology with quantitative electron microscopy, we have attempted to correlate the inhibition of neurotransmitter release with lesions in particular stages of the synaptic vesicle cycle. Two of the inhibitory Rabphilin-3A constructs, the full-length protein and the 1–280 fragment, affected the distribution of membrane in the endocytotic pathway involved in recycling of synaptic vesicle components. While the inhibitory 396–704 fragment had no effect on endocytotic compartments, the exocytotic compartments (number and spatial distribution of synaptic vesicles) of terminals injected with the 396–704 and 1–280 fragments were almost identical. The differential actions of these various Rabphilin-3A constructs on exocytosis and endocytosis indicate separate and distinct roles for Rabphilin-3A in the exocytotic and endocytotic pathways. Therefore, we conclude that Rabphilin-3A is a multifunctional coordinator of synaptic vesicle traffic.
Rabphilin-3A Regulates Neurotransmitter Release
Half-maximal inhibition of transmitter release required ∼0.5 μM Rabphilin-3A, which is similar to the dissociation constant of 0.2 μM reported for Rabphilin-3A binding to Rab3A in vitro (Yamaguchi et al., 1993). This suggests that the concentrations that we injected are relevant to the in vivo biochemistry of the presynaptic terminal. The potent inhibitory effects of the NH2-terminal domain presumably are specific to Rabphilin-3A, because this domain does not resemble any other known presynaptic protein. However, because the COOH-terminal domain of Rabphilin-3A has some sequence overlap with other C2 domain proteins, such as protein kinase C, synaptotagmin, and Doc2 (reviewed by Südhof and Rizo, 1996), it is possible that the C2 domains of Rabphilin-3A may have acted by perturbing the function of these other C2 domain proteins. Although we cannot formally exclude this possibility, several observations argue against it. First, members of the C2 protein family each appear to bind to unique sets of proteins (Südhof and Rizo, 1996). Second, injection of higher concentrations of Doc2, which also contains two C2 domains, had no effect on neurotransmitter release. Third, both the COOH- and the NH2-terminal fragments of Rabphilin-3A had similar effects on the spatial distribution of synaptic vesicles. Thus, it is likely that the actions of our reagents are due to specific perturbation of Rabphilin-3A.
Previous work has shown that introduction of Rabphilin-3A into chromaffin cells stimulates secretion (Chung et al., 1995) and has no effect on exocytosis in PC12 cells (Komuro et al., 1996) or mouse eggs (Masumoto et al., 1996). It is not yet possible to account for the diverse actions of full-length Rabphilin-3A in different secretory systems. One possibility is that long-term manipulations of Rabphilin-3A, such as those performed in chromaffin and pheochromocytoma (PC12) cells, may induce compensatory mechanisms that are avoided by the acute manipulations we have made (Augustine et al., 1996). For example, genetic knock out of the Rab3A gene of mice affects the expression and localization of Rabphilin-3A in neurons (Li et al., 1994), yet has relatively little effect on transmitter release (Geppert et al., 1994; Weber et al., 1996). An additional possibility is that the relative expression levels of Rabphilin-3A and its binding partners may be an important variable in determining the response to exogenous Rabphilin-3A. Finally, because the calcium signaling pathways of these various cell types differ (Augustine and Neher, 1992; Neher and Zucker, 1993; Heidelberger et al., 1994; Hsu et al., 1996), a calcium-binding protein such as Rabphilin-3A may play somewhat different roles in different cell types. Although these factors make it difficult to compare results obtained in these different experimental systems, our experiments are the first investigation into the role of Rabphilin-3A in neurons and clearly indicate that acute introduction of Rabphilin-3A potently inhibits synaptic release of neurotransmitter.
Functional Domains of Rabphilin-3A
Microinjection of full-length Rabphilin-3A and two Rabphilin-3A protein fragments each produced a potent, dose-dependent inhibition of neurotransmitter release. The design of our experiment is based on the assumption that the individual fragments act as competitive inhibitors of endogenous Rabphilin-3A. From this standpoint, our findings that injection of either full-length Rabphilin-3A or its individual fragments inhibited neurotransmitter release could suggest the surprising conclusion that both reagents caused identical perturbations, either a gain- or loss-of-function phenotype. However, the structural effects of the fragments and full-length Rabphilin-3A are separate and distinct. Thus, while both categories of reagents inhibit release, we conclude that they inhibit via different mechanisms that are consistent with the basic design assumption.
The actions of the various Rabphilin-3A fragments define two of the structural domains that are necessary for Rabphilin-3A function. One domain is within residues 1–280, which contains the Rab3A binding site and the sites for phosphorylation by calmodulin-dependent protein kinase II and cAMP-dependent kinase (Kato et al., 1994; Numata et al., 1995; Fyske et al., 1995). NH2-terminal fragments of Rabphilin-3A also inhibit exocytosis in chromaffin cells, PC12 cells, and mouse eggs (Chung et al., 1995; Komuro et al., 1996; Masumoto et al., 1996). Future experiments will be needed to determine whether the Rab3A-binding domain, the phosphorylation domain, or some other locus is responsible for the inhibitory action of this fragment.
The second region that appears to be critical for Rabphilin-3A function contains the C2 domains (residues 396–704). Our findings are consistent with the effect of a similar fragment (residues 403–704) on exocytosis in PC12 cells (Komuro et al., 1996) and mouse eggs (Masumoto et al., 1996). The C2 domains are known to bind calcium, phospholipid, and the cytoskeletal protein β-adducin; the inhibitory action of this fragment suggests that one or more of these interactions might be required for the function of Rabphilin-3A in neurotransmitter release. However, it is curious that the larger 281–704 fragment, which contains the active 396–704 domain, was completely inactive. This longer fragment also has no effect in PC12 cells or chromaffin cells (Chung et al., 1995; Komuro et al., 1996). Together, these data suggest that the central region of the larger fragment (281–395) may act as an autoinhibitory domain that regulates the binding interactions of the C2 domains. Alternatively, the longer fragment simply may lack the conformational requirements for binding to its targets inside the cell.
Rabphilin-3A Regulates Endocytosis
Several of our results indicate that Rabphilin-3A plays a role in endocytosis. Injecting full-length Rabphilin-3A increased the membrane area of vesicular endosomes and caused a commensurate decrease in the area of membrane associated with coated vesicles and the plasma membrane. Manipulations that promote endocytotic traffic, such as perturbations of GTP-binding proteins, frequently cause endosomes to appear as large, empty vesicles (Bucci et al., 1992; Brady et al., 1993; DeHoop et al., 1994; Mundigl et al., 1993). Therefore, the simplest interpretation of our data is that Rabphilin-3A promoted the retrieval of membrane from the plasma membrane, which then accumulated in endosomal compartments. In contrast, the 1–280 fragment caused a decrease in the membrane areas of synaptic and coated vesicles and an expansion of the area of the plasma membrane, suggesting that this fragment prevented membrane retrieval from the plasma membrane by acting as a competitive inhibitor of Rabphilin-3A. Since Rabphilin-3A is not associated with clathrin-coated vesicles (Stahl et al., 1996), it appears that the NH2 terminus of Rabphilin-3A regulates an early step of membrane retrieval that (a) precedes coated vesicle formation, and (b) leads to vesicular endosomes.
The protein–protein interactions that mediate the endocytotic actions of Rabphilin-3A are not yet clear. Although the NH2 terminus of Rabphilin-3A binds Rab3A, it is generally acknowledged that Rab3A does not participate in endocytosis (Simons and Zerial, 1993; Takai et al., 1996; Südhof, 1997). Because the NH2 terminus of Rabphilin-3A also binds to α-actinin (Miyazaki et al., 1994), which participates in actin filament bundling, this domain could provide a link between the synaptic vesicle and the cytoskeleton that is required for endocytosis.
How Does Rabphilin-3A Regulate Exocytosis?
The inhibition of transmitter release produced by injection of Rabphilin-3A and its individual domains is not solely the result of an inhibition of endocytosis. For all inhibitory constructs, vesicles were present at the active zones despite complete inhibition of transmitter release. Each inhibitory fragment caused a relative increase in the number of synaptic vesicles 50–100 nm from the plasma membrane. Particularly dramatic was the effect of injecting full-length Rabphilin-3A, which inhibited release without affecting the number or spatial distribution of synaptic vesicles (Fig. 3). By what mechanism do these reagents prevent vesicles from fusing with the plasma membrane in response to an action potential?
Our working hypothesis is that Rabphilin-3A is involved in both docking and priming of synaptic vesicles. We propose that the fragments of Rabphilin-3A, which should act as competitive inhibitors of Rabphilin-mediated interactions, inhibit the docking reaction. This would account for the observed relative accumulation of undocked vesicles after injection of these fragments. In contrast, we propose that excess full-length Rabphilin-3A can function in the docking reaction, but prevents the priming reaction, perhaps by continuing to bind to Rab3A and preventing GTP hydrolysis (Kishida et al., 1993). This block of vesicle priming could account for our observation that injecting full-length protein inhibits exocytosis at a stage of the vesicle cycle that is morphologically indistinct, as priming may be (Parsons et al., 1995).
If priming involves an increased binding affinity of the vesicle for the plasma membrane, perturbations of vesicle priming might not affect the observed number of docked vesicles, whereas those that act after priming cause an accumulation of fusion-competent vesicles at the plasma membrane. Indeed, this model can rationalize otherwise puzzling observations that some perturbations of SNARE complex proteins (Söllner et al., 1993; Schiavo et al., 1995), such as synaptotagmin, synaptobrevin, SNAP, and syntaxin, inhibit exocytosis and result in an accumulation of docked vesicles (Bommert et al., 1993; Hunt et al., 1994; DeBello et al., 1995; Broadie et al., 1995), while other SNARE perturbations (syntaxin, unc-18, SNAP-25) that inhibit neurotransmitter release have no effect on the number of docked synaptic vesicles (O'Connor et al., 1997; Dresbach et al., 1998; Burns, 1996). Thus, we propose that the number of docked synaptic vesicles can be used to differentiate between a perturbation that acts upstream and one that acts downstream of vesicle priming. More work is needed to test the validity of this hypothesis.
C2 Domain Proteins as Multifunctional Coordinators of Membrane Traffic
In summary, our data indicate that Rabphilin-3A is involved in both endocytosis and exocytosis of the synaptic vesicle cycle underlying neurotransmitter release, consistent with the multiple biochemical properties of Rabphilin-3A. What is the advantage of having such a multifunctional protein? One of the most striking features of the synaptic vesicle cycle is that exocytosis and endocytosis are coupled to maintain a complement of synaptic vesicles at each active zone. Involving a calcium-binding protein such as Rabphilin-3A in both endocytosis and exocytosis may simultaneously regulate both and thereby coordinate vesicle pool size. It should be noted that other C2 domain proteins, particularly synaptotagmin (Jorgensen et al., 1995; Ullrich et al., 1994; Zhang et al., 1994; Fukuda et al., 1995) and protein kinase C (Felder et al., 1992; Robinson et al., 1993; Gillis et al., 1996), also have been implicated in both exocytosis and endocytosis. Thus, C2 domain proteins may serve as an entire family of multifunctional regulators of synaptic vesicle traffic.
Acknowledgments
We thank Nikon Inc. and Noran Instruments for the generous loan of microscopes, and P. Bent, W. Hausner, and A. Meyerov for their assistance in making these systems available to us. We are particularly grateful to L. Hawkey for his EM expertise. We also thank F.E. Schweizer and T. Blandpied for commenting on the manuscript, M. Helms and M. Rose for technical support, and L. Parker for participating in experiments.
This study was supported by National Institutes of Health grants MH-11073 and NS-21624, and Sigma Xi Grant-in-Aid of Research.
Abbreviation used in this paper
- PSP
postsynaptic potential
references
- Augustine GJ, Burns ME, DeBello WM, Pettit DL, Schweizer FE. Exocytosis: proteins and perturbations. Annu Rev Pharmacol Toxicol. 1996;36:659–701. doi: 10.1146/annurev.pa.36.040196.003303. [DOI] [PubMed] [Google Scholar]
- Augustine GJ, Charlton MP, Horn R. Role of calcium-activated potassium channels in transmitter release and the squid giant synapse. J Physiol (Camb) 1988;398:149–164. doi: 10.1113/jphysiol.1988.sp017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ, Charlton MP, Smith SJ. Calcium entry into voltage-clamped presynaptic terminals of squid. J Physiol (Camb) 1985;367:143–162. doi: 10.1113/jphysiol.1985.sp015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ, Eckert R. Divalent cations differentially support transmitter release at the squid giant synapse. J Physiol (Camb) 1984;346:257–271. doi: 10.1113/jphysiol.1984.sp015020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ, Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol (Camb) 1992;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommert K, Charlton MP, DeBello WM, Chin GJ, Betz H, Augustine GJ. Inhibition of neurotransmitter release by C2-domain peptides implicates synaptotagmin in exocytosis. Nature. 1993;363:163–165. doi: 10.1038/363163a0. [DOI] [PubMed] [Google Scholar]
- Brady ST, Sugimori M, Leopold PL, Lin J-W, Chu DS, Llinás R. Activity-dependent inhibition of neurotransmitter release by brefeldin A. Biol Bull (Woods Hole) 1993;185:299–300. doi: 10.1086/BBLv185n2p299. [DOI] [PubMed] [Google Scholar]
- Broadie K, Prokop A, Bellen HJ, O'Kane CJ, Schulze KL, Sweeney ST. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila. . Neuron. 1995;15:663–673. doi: 10.1016/0896-6273(95)90154-x. [DOI] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase Rab5a functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Burns, M.E. 1996. Molecular Mechanisms of Neurotransmitter Release: The Functions of Rab3A, Rabphilin and SNAP-25. Doctoral dissertation, Duke University.
- Burns ME, Beushausen SA, Chin GJ, Tang D, DeBello WM, Dresbach T, O'Connor V, Schweizer FE, Wang SS-H, Whiteheart SW, et al. Proteins involved in synaptic vesicle docking and fusion. Cold Spring Harbor Symp Quant Biol. 1996;60:337–348. doi: 10.1101/sqb.1995.060.01.038. [DOI] [PubMed] [Google Scholar]
- Charlton MP, Atwood HL. Slow release of transmitter at the squid giant synapse. Neurosci Lett. 1977;5:165–169. doi: 10.1016/0304-3940(77)90041-6. [DOI] [PubMed] [Google Scholar]
- Charlton MP, Smith SJ, Zucker RS. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol (Camb) 1982;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S-H, Takai Y, Holz RW. Evidence that the Rab3A-binding protein, Rabphilin-3A, enhances regulated secretion. J Biol Chem. 1995;270:16714–16718. doi: 10.1074/jbc.270.28.16714. [DOI] [PubMed] [Google Scholar]
- Couteaux R, Pecot-Dechavassine M. Les zones specialisees des membranes presynaptiques. C R Acad Sci Hebd Seances Acad Sci D. 1974;278:291–293. [PubMed] [Google Scholar]
- DeCamilli P. Molecular mechanisms in synaptic vesicle cycling. FEBS Lett. 1995;369:3–12. doi: 10.1016/0014-5793(95)00739-v. [DOI] [PubMed] [Google Scholar]
- DeBello WM, Betz H, Augustine GJ. Synaptotagmin and neurotransmitter release. Cell. 1993;74:947–950. doi: 10.1016/0092-8674(93)90716-4. [DOI] [PubMed] [Google Scholar]
- DeBello WM, O'Connor V, Dresbach T, Whiteheart SW, Wang SS-H, Schweizer FE, Betz H, Rothman JE, Augustine GJ. SNAP-mediated protein–protein interactions essential for neurotransmitter release. Nature. 1995;373:626–630. doi: 10.1038/373626a0. [DOI] [PubMed] [Google Scholar]
- De Hoop MJ, Huber LA, Stenmark H, Williamson E, Zerial M, Parton RG, Dotti CG. The involvement of the small GTP-binding protein Rab5a in neuronal endocytosis. Neuron. 1994;13:11–22. doi: 10.1016/0896-6273(94)90456-1. [DOI] [PubMed] [Google Scholar]
- Dresbach, T., M.E. Burns, V. O'Connor, W.M. De Bello, H. Betz, and G.J. Augustine. 1998. A neuronal Sec-1 homologue regulates neurotransmitter release at the squid giant synapse. J. Neurosci. In press. [DOI] [PMC free article] [PubMed]
- Felder S, LaVin J, Ullrich A, Schlessinger J. Kinetics of binding, endocytosis, and recycling of EGF receptor mutants. J Cell Biol. 1992;117:203–212. doi: 10.1083/jcb.117.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Moreira FMT, Lewis M, Sugimori M, Niiboe K, Mikoshiba K, Llinás R. Role of the C2B domain of synaptotagmin in vesicular release and recycling as determined by specific antibody injection into the squid giant synapse. Proc Natl Acad Sci USA. 1995;92:10708–10712. doi: 10.1073/pnas.92.23.10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyske EM, Li C, Südhof TC. Phosphorylation of Rabphilin-3A3a by calcium/calmodulin- and cAMP-dependent protein kinases in vitro. J Neurosci. 1995;15:2385–2395. doi: 10.1523/JNEUROSCI.15-03-02385.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, DeCamilli P, Hammer RE, Südhof TC. The role of Rab3A in neurotransmitter release. Nature. 1994;369:493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- Gillis KD, Mossner R, Neher E. Protein kinase C enhances exocytosis from chromaffin cells by increasing the size of the readily releasable pool of secretory granules. Neuron. 1996;16:1209–1220. doi: 10.1016/s0896-6273(00)80147-6. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Hess SD, Doroshenko PA, Augustine GJ. A functional role for GTP-binding proteins in synaptic vesicle cycling. Science. 1993;259:1169–1172. doi: 10.1126/science.8438167. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS, Dennis MJ, Jan Y, Jan L, Evans L. Synaptic vesicle exocytosis captured by quickfreezing and correlated with quantal transmitter release. J Cell Biol. 1979;81:275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S-F, Augustine GJ, Jackson MB. Adaptation of calcium-triggered exocytosis in presynaptic terminals. Neuron. 1996;17:501–512. doi: 10.1016/s0896-6273(00)80182-8. [DOI] [PubMed] [Google Scholar]
- Hughes CA, Bennett V. Adducin: a physical model with implications for function in assembly of spectrin-actin complexes. J Biol Chem. 1995;270:18990–18996. doi: 10.1074/jbc.270.32.18990. [DOI] [PubMed] [Google Scholar]
- Hunt JM, Bommert K, Charlton MP, Kistner A, Habermann E, Betz H, Augustine GJ. A post-docking role for synaptobrevin in synaptic vesicle fusion. Neuron. 1994;12:1269–1279. doi: 10.1016/0896-6273(94)90443-x. [DOI] [PubMed] [Google Scholar]
- Jorgensen EM, Hartwig E, Schuske K, Nonet ML, Jin Y, Horvitz HR. Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans. . Nature. 1995;378:196–199. doi: 10.1038/378196a0. [DOI] [PubMed] [Google Scholar]
- Kato M, Sasaki T, Imazumi K, Takahashi K, Araki K, Shirataki H, Matsuura Y, Ishida A, Fujisawa H, Takai Y. Phosphorylation of Rabphilin-3A by calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1994;205:1776–1844. doi: 10.1006/bbrc.1994.2875. [DOI] [PubMed] [Google Scholar]
- Kato M, Sasaki T, Ohya T, Nakanishi H, Nishioka H, Imamura M, Takai Y. Physical and functional interaction of Rabphilin-3A with alpha-actinin. J Biol Chem. 1996;271:31775–31778. doi: 10.1074/jbc.271.50.31775. [DOI] [PubMed] [Google Scholar]
- Katz, B. 1966. Nerve, Muscle, and Synapse. New York: McGraw-Hill.
- Kelly RB. Storage and release of neurotransmitters. Cell. 1993a;72:43–53. doi: 10.1016/s0092-8674(05)80027-3. [DOI] [PubMed] [Google Scholar]
- Kelly RB. A question of endosomes. Nature. 1993b;364:487–488. doi: 10.1038/364487a0. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Nakanishi H, Takai Y. Purification and properties of Rab3A. Methods Enzymol. 1995;257:57–70. doi: 10.1016/s0076-6879(95)57010-1. [DOI] [PubMed] [Google Scholar]
- Kishida S, Shirataki H, Sasaki T, Kato M, Kaibuchi K, Takai Y. Rab3A GTPase-activating protein-inhibiting activity of Rabphilin-3A3a, a putative Rab3A target protein. J Biol Chem. 1993;268:22259–22261. [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Synaptic vesicles have two distinct recycling pathways. J Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro R, Sasaki T, Orita S, Maeda M, Takai Y. Involvement of Rabphilin-3A3a in Ca-dependent exocytosis from PC12 cells. Biophys Biochem Res Commun. 1996;219:435–440. doi: 10.1006/bbrc.1996.0251. [DOI] [PubMed] [Google Scholar]
- Li C, Takei K, Geppert M, Daniell L, Steinus K, Chapman ER, Jahn R, DeCamilli P, Südhof TC. Synaptic targeting of Rabphilin-3A3a, a synaptic vesicle Ca/phospholipid-binding protein, depends on Rab3A/3C. Neuron. 1994;13:885–898. doi: 10.1016/0896-6273(94)90254-2. [DOI] [PubMed] [Google Scholar]
- Mann DW, Joyner RW. Miniature synaptic potentials at the squid giant synapse. J Neurobiol. 1978;94:329–335. doi: 10.1002/neu.480090410. [DOI] [PubMed] [Google Scholar]
- Martin R, Miledi R. The form and dimensions of the giant synapse of squids. Philos Trans R Soc Lond B Biol Sci. 1986;312:355–377. [Google Scholar]
- Masumoto N, Sasaki T, Tahara M, Mammoto A, Ikebuchi Y, Tasaka K, Tokunaga M, Takai Y, Miyake A. Involvement of Rabphilin-3A in cortical granule exocytosis in mouse eggs. J Cell Biol. 1996;135:1741–1747. doi: 10.1083/jcb.135.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan CJ, Stabila PF, Macara IG. Role of the Rab3A-binding domain in targeting of Rabphilin-3A to vesicle membranes of PC12 cells. Mol Cell Biol. 1996;16:4985–4995. doi: 10.1128/mcb.16.9.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miche SM, Mooseker MS, Morrow JS. Erythrocyte adducin: a calmodulin-regulated actin-bundling protein that stimulates spectrin-actin binding. J Cell Biol. 1987;105:2837–2845. doi: 10.1083/jcb.105.6.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Spontaneous synaptic potentials and quantal release of transmitter in the stellate ganglia of the squid. J Physiol (Camb) 1967;192:379–406. doi: 10.1113/jphysiol.1967.sp008306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973;183:173–193. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Miller TM, Heuser JE. Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J Cell Biol. 1984;98:685–698. doi: 10.1083/jcb.98.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Shirataki H, Kohno H, Kaibuchi K, Tsugita A, Takai Y. Identification as β-adducin of a protein interacting with Rabphilin-3A3a in the presence of calcium and phosphatidylserine. Biochem Biophys Res Commun. 1994;205:460–466. doi: 10.1006/bbrc.1994.2688. [DOI] [PubMed] [Google Scholar]
- Mundigl O, Matteoli M, Danielle L, Thomas-Reetz A, Metcalf A, Jahn R, DeCamilli P. Synaptic vesicle proteins and early endosomes in cultured hippocampal neurons: differential effects of brefeldin A in axon and dendrites. J Cell Biol. 1993;122:1207–1221. doi: 10.1083/jcb.122.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Zucker RS. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993;10:21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- Numata S, Shirataki H, Hagi S, Yamamoto T, Takai Y. Phosphorylation of Rabphilin-3A, a putative target protein for Rab3A, by cyclic AMP-dependent protein kinase. Biochem Biophys Res Commun. 1995;203:1927–1934. doi: 10.1006/bbrc.1994.2413. [DOI] [PubMed] [Google Scholar]
- O'Connor V, Heuss C, DeBello WM, Dresbach T, Charlton MP, Hunt JM, Pellegrini L, Hodel A, Burger MM, Betz H, et al. Disruption of syntaxin-mediated protein interactions blocks neurotransmitter secretion. Proc Natl Acad Sci USA. 1997;94:12186–12190. doi: 10.1073/pnas.94.22.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Coorssen JR, Horstmann H, Almers W. Docked granules, the exocytic burst and the need for ATP hydrolysis in endocrine cells. Neuron. 1995;15:1085–1096. doi: 10.1016/0896-6273(95)90097-7. [DOI] [PubMed] [Google Scholar]
- Robinson PJ, Sontag JM, Liu JP, Fyske EM, Slaughter C, McMahon H, Südhof TC. Dynamin GTPase regulated by protein kinase C phosphorylation in nerve terminals. Nature. 1993;365:163–166. doi: 10.1038/365163a0. [DOI] [PubMed] [Google Scholar]
- Sanchez ME, Nuno CM, Buchanan J, Augustine GJ. Contractions of the squid stellate ganglion. J Exp Biol. 1990;152:369–387. doi: 10.1242/jeb.152.1.369. [DOI] [PubMed] [Google Scholar]
- Scheller RH. Membrane trafficking in the presynaptic nerve terminal. Neuron. 1995;14:689–696. doi: 10.1016/0896-6273(95)90328-3. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Gmachi MJS, Stenbeck G, Söllner TH, Rothman JE. A possible docking and fusion particle for synaptic transmission. Nature. 1995;378:733–736. doi: 10.1038/378733a0. [DOI] [PubMed] [Google Scholar]
- Schweizer FE, Betz H, Augustine GJ. From vesicle docking to endocytosis: intermediate reactions of exocytosis. Neuron. 1995;14:689–696. doi: 10.1016/0896-6273(95)90213-9. [DOI] [PubMed] [Google Scholar]
- Schweizer, F.E., T. Dresbach, W.M. DeBello, V. O'Connor, G.J. Augustine, and H. Betz. 1998. Regulation of neurotransmitter release kinetics by NSF. Science. In press. [DOI] [PubMed]
- Shirataki H, Kaibuchi K, Sakoda T, Kishida S, Yamaguchi T, Wada K, Miyazaki M, Takai Y. Rabphilin-3A3a, a putative target protein for smgp25a/Rab3Ap25 small GTP-binding protein related to synaptotagmin. Mol Cell Biol. 1993;13:2061–2068. doi: 10.1128/mcb.13.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirataki H, Takai Y. Purification and properties of Rabphilin-3A. Methods Enzymol. 1995;257:291–302. doi: 10.1016/s0076-6879(95)57033-0. [DOI] [PubMed] [Google Scholar]
- Shirataki H, Yamamoto T, Hagi S, Miura H, Oishi H, Jin-no Y, Sebonmatsu T, Takai Y. Rabphilin-3A is associated with synaptic vesicles through a vesicle protein in a manner independent of Rab3A. J Biol Chem. 1994;269:32717–32720. [PubMed] [Google Scholar]
- Simons K, Zerial M. Rab proteins and the road maps for intracellular transport. Neuron. 1993;11:789–799. doi: 10.1016/0896-6273(93)90109-5. [DOI] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly–disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Stahl B, Chou JH, Li C, Südhof TC, Jahn R. Rab3 reversibly recruits Rabphilin-3A to synaptic vesicles by a mechanism analogous to raf recruitment by ras. EMBO (Eur Mol Biol Organ) J. 1996;15:1799–1809. [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Südhof TC. Function of Rab3 GDP-GTP exchange. Neuron. 1997;18:519–522. doi: 10.1016/s0896-6273(00)80292-5. [DOI] [PubMed] [Google Scholar]
- Südhof TC, Rizo J. Synaptotagmins: C2 domain proteins that regulate synaptic vesicle traffic. Neuron. 1996;17:379–388. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Shirataki H, Nakanishi H. Rab3A small GTP-binding protein in calcium-dependent exocytosis. Genes Cells. 1996;1:615–632. doi: 10.1046/j.1365-2443.1996.00257.x. [DOI] [PubMed] [Google Scholar]
- Takei K, Mundigl O, Daniell L, DeCamilli P. The synaptic vesicle cycle: a single vesicle budding step involving clathrin and dynamin. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torri-Tarelli F, Haimann C, Ceccarelli B. Coated vesicles and pits during enhanced quantal release of acetylcholine at the neuromuscular junction. J Neurocytol. 1987;16:205–214. doi: 10.1007/BF01795304. [DOI] [PubMed] [Google Scholar]
- Ullrich B, Li C, Zhang JZ, McMahon H, Anderson RG, Geppert M, Südhof TC. Functional properties of multiple synaptotagmins in brain. Neuron. 1994;13:1281–1291. doi: 10.1016/0896-6273(94)90415-4. [DOI] [PubMed] [Google Scholar]
- Weber E, Jilling T, Kirk KL. Distinct functional properties of Rab3A and Rab3B in PC12 neuroendocrine cells. J Biol Chem. 1996;271:6963–6971. doi: 10.1074/jbc.271.12.6963. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Shirataki H, Kishida S, Miyazaki M, Nishikawa J, Wada K, Numata S, Kaibuchi K, Takai Y. Two functionally different domains of Rabphilin-3A3a, Rab3A p25/smg p25A-binding and phospholipid- and Ca-binding domains. J Biol Chem. 1993;268:27164–27170. [PubMed] [Google Scholar]
- Zhang JZ, Davletov BA, Südhof TC, Anderson RGW. Synaptotagmin I is a high affinity receptor for Clathrin AP-2: implications for membrane recycling. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]