Abstract
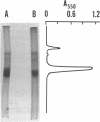
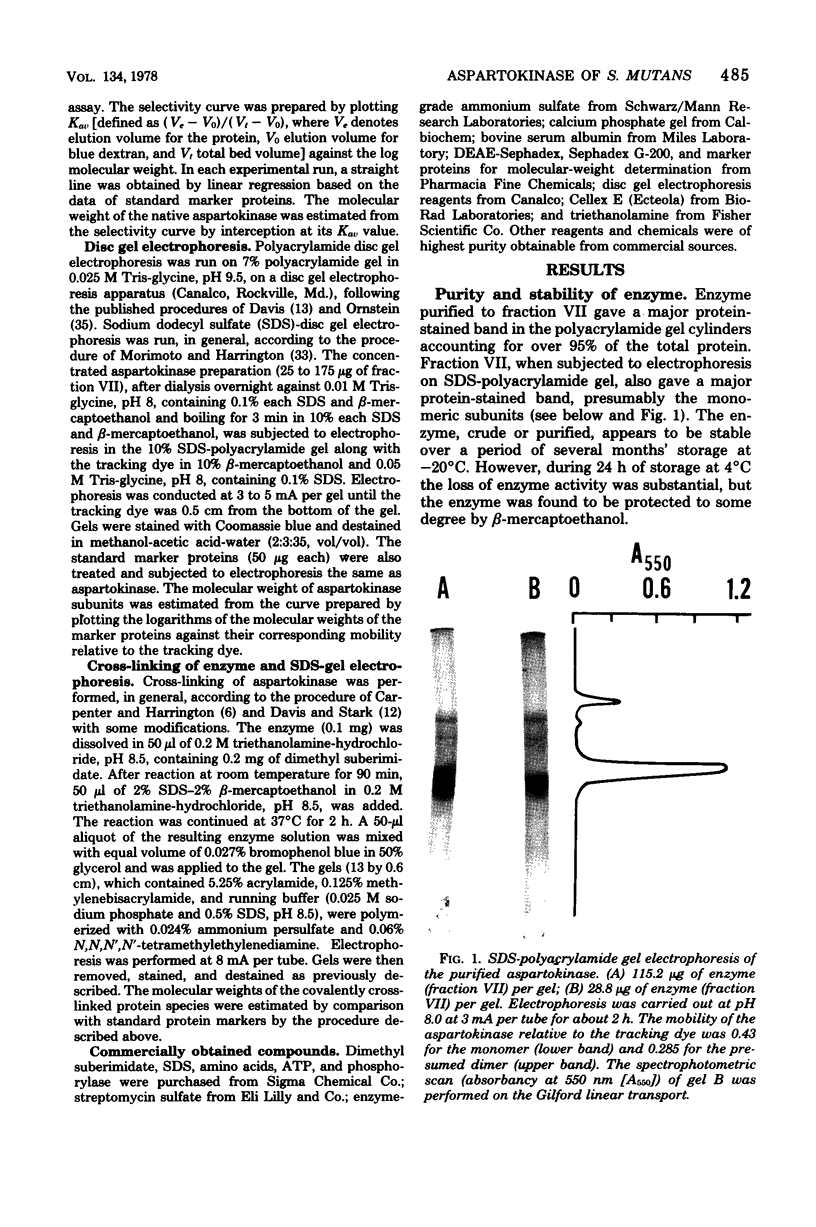
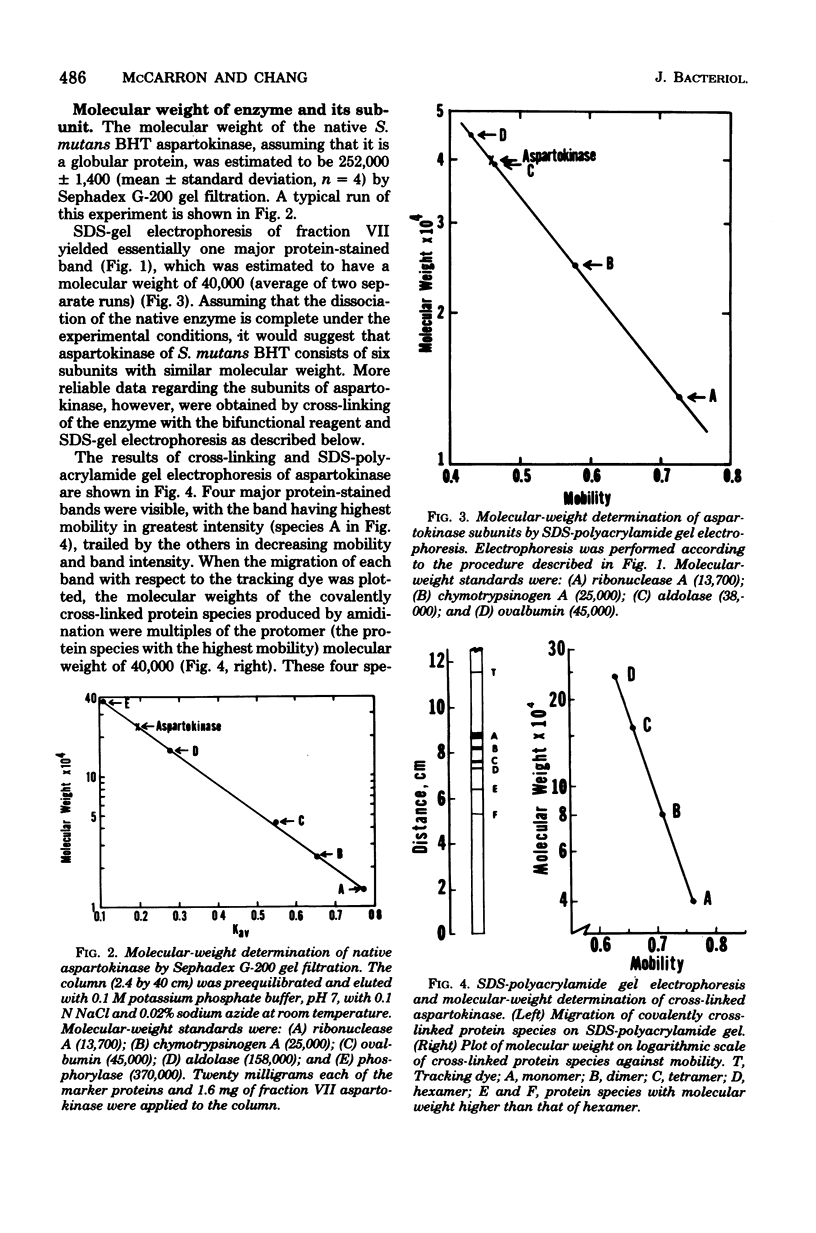
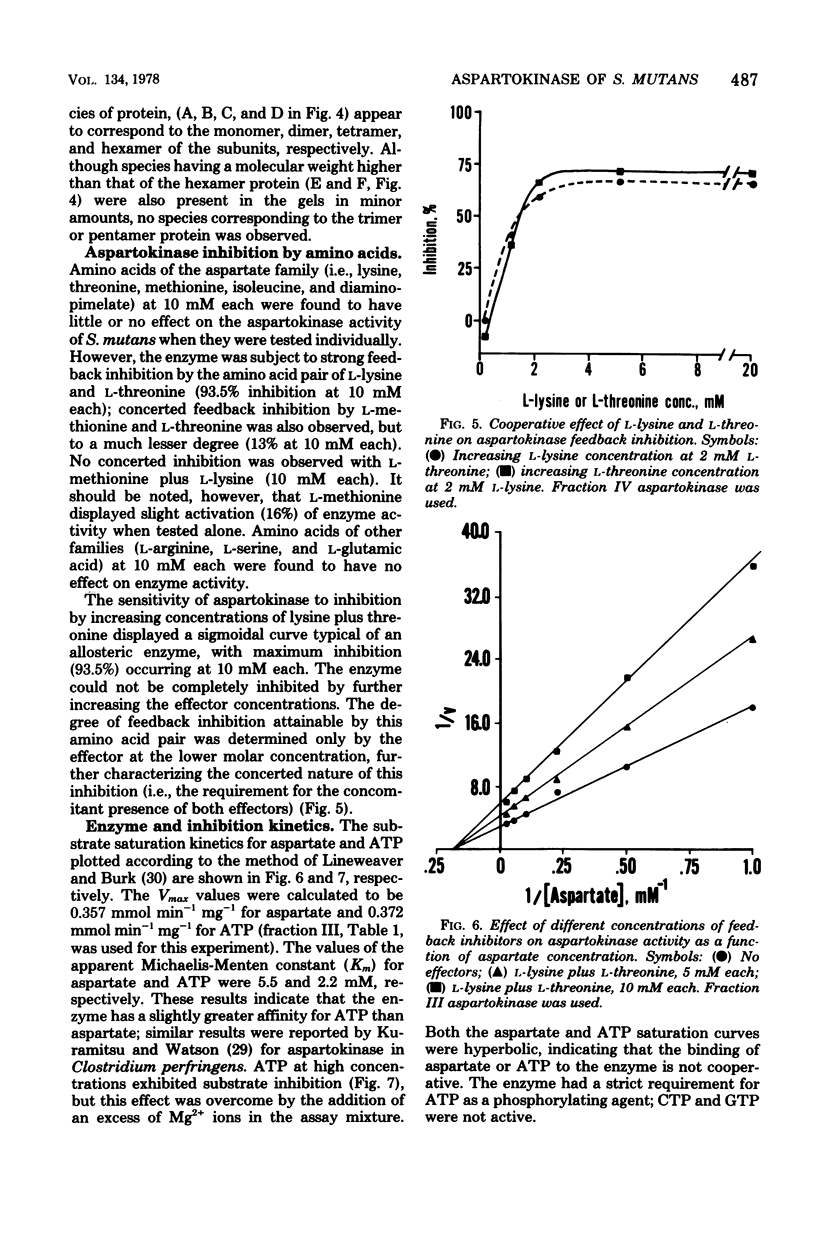
Aspartokinase from Streptococcus mutans BHT was purified to homogeneity and characterized. The molecular weight of the native enzyme was estimated to be 242,000 by gel filtration. Cross-linking of aspartokinase with dimethyl suberimidate and polyacrylamide gel electrophoresis of the amidinated enzyme in the presence of sodium dodecyl sulfate showed the enzyme to be composed of six identical subunits with a molecular wieght of 40,000. The optimal pH range for enzyme activity was 6.5 to 8.5. The apparent Michaelis-Menten constants for aspartate and ATP were 5.5 and 2.2 mM, respectively. The enzyme was stable within the temperature range of 10 to 35 degrees C. Aspartokinase was not feedback inhibited by individual amino acids, but was concertedly inhibited by L-lysine and L-threonine (93.5% inhibition at 10 mM each). The inhibition was noncompetitive with respect to aspartate (Ki = 10 mM) and mixed with respect to ATP. L-Threonine methyl ester and L-threonine amide were able to substitute for L-threonine in feedback inhibition, but the requirement for L-lysine uas strict. The feedback inhibitor pair protected the enzyme against heat denaturation. Aspartokinase synthesis was repressed by L-threonine; this repression was enhanced by L-lysine, but was slightly attenuated by L-methionine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong W. G. The composition of organic films formed on human teeth. Caries Res. 1967;1(2):89–103. doi: 10.1159/000259504. [DOI] [PubMed] [Google Scholar]
- BATTISTONE G. C., BURNETT G. W. The free amino acid composition of human saliva. Arch Oral Biol. 1961 Apr;3:161–170. doi: 10.1016/0003-9969(61)90133-9. [DOI] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- CHANGEUX J. P. The feedback control mechanisms of biosynthetic L-threonine deaminase by L-isoleucine. Cold Spring Harb Symp Quant Biol. 1961;26:313–318. doi: 10.1101/sqb.1961.026.01.037. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Nutritional requirements of Streptococcus sanguis. Arch Oral Biol. 1972 Sep;17(9):1327–1332. doi: 10.1016/0003-9969(72)90165-3. [DOI] [PubMed] [Google Scholar]
- Carpenter F. H., Harrington K. T. Intermolecular cross-linking of monomeric proteins and cross-linking of oligomeric proteins as a probe of quaternary structure. Application to leucine aminopeptidase (bovine lens). J Biol Chem. 1972 Sep 10;247(17):5580–5586. [PubMed] [Google Scholar]
- Cowman R. A., Perrella M. M., Fitzgerald R. J. Influence of incubation atmosphere on growth and amino acid requirements of Streptococcus mutans. Appl Microbiol. 1974 Jan;27(1):86–92. doi: 10.1128/am.27.1.86-92.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley P. The breakdown of the carbohydrate and protein matrix of dental plaque. Caries Res. 1969;3(3):249–265. doi: 10.1159/000259599. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P., Prakash L. Aspartokinase of Rhodopseudomonas spheroides. Regulation of enzyme activity by aspartate beta-semialdehyde. J Biol Chem. 1966 Dec 25;241(24):5827–5835. [PubMed] [Google Scholar]
- Datta P. Regulation of branched biosynthetic pathways in bacteria. Science. 1969 Aug 8;165(3893):556–562. doi: 10.1126/science.165.3893.556. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer Dirks O. The relationship between extracellular polysaccharide-producing streptococci and smooth surface caries in 13-year-old children. Caries Res. 1969;3(2):190–199. doi: 10.1159/000259582. [DOI] [PubMed] [Google Scholar]
- Dungan S. M., Katta P. Concerted feedback inhibition. Purification and some properties of aspartokinase from Pseudomonas fluorescens. J Biol Chem. 1973 Dec 25;248(24):8534–8540. [PubMed] [Google Scholar]
- Filer D., Kindler S. H., Rosenberg E. Aspartokinase of Micrococcus luteus. 'Feedback-stimulation" by methionine. FEBS Lett. 1977 Mar 15;75(1):177–179. doi: 10.1016/0014-5793(77)80080-x. [DOI] [PubMed] [Google Scholar]
- Fitzgerlad R. J. Dental caries research in gnotobiotic animals. Caries Res. 1968;2(2):139–146. doi: 10.1159/000259552. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Depaola P. F., Spinell D. M., Skobe Z. Interdental localization of Streptococcus mutans as related to dental caries experience. Infect Immun. 1974 Mar;9(3):481–488. doi: 10.1128/iai.9.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold O. G., Jordan H. V., Van Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973 Nov;18(11):1357–1364. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- Hitchcock M. H., Hodgson B. Lysine- and lysine-plus-threonine-inhibitable aspartokinases in Bacillus brevis. Biochim Biophys Acta. 1976 Sep 14;445(2):350–363. doi: 10.1016/0005-2744(76)90089-9. [DOI] [PubMed] [Google Scholar]
- Hyatt A. T., Hayes M. L. Free amino acids and amines in human dental plaque. Arch Oral Biol. 1975 Mar;20(3):203–209. doi: 10.1016/0003-9969(75)90010-2. [DOI] [PubMed] [Google Scholar]
- Keyes P. H. Research in dental caries. J Am Dent Assoc. 1968 Jun;76(6):1357–1373. doi: 10.14219/jada.archive.1968.0186. [DOI] [PubMed] [Google Scholar]
- Krasse B., Jordan H. V., Edwardsson S., Svensson I., Trell L. The occurrence of certain "caries-inducing" streptococci in human dental plaque material with special reference to frequency and activity of caries. Arch Oral Biol. 1968 Aug;13(8):911–918. doi: 10.1016/0003-9969(68)90006-x. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Concerted feedback inhibition of aspartokinase from Bacillus stearothermophilus. I. Catalytic and regulatory properties. J Biol Chem. 1970 Jun 10;245(11):2991–2997. [PubMed] [Google Scholar]
- Kuramitsu H. K., Watson R. M. Regulation of aspartokinase activity in Clostridium perfringens. J Bacteriol. 1973 Sep;115(3):882–888. doi: 10.1128/jb.115.3.882-888.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morimoto K., Harrington W. F. Evidence for structural changes in vertebrate thick filaments induced by calcium. J Mol Biol. 1974 Sep 25;88(3):693–709. doi: 10.1016/0022-2836(74)90417-3. [DOI] [PubMed] [Google Scholar]
- Newbrun E. Sucrose, the arch criminal of dental caries. Odontol Revy. 1967;18(4):373–386. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Paulus H., Gray E. Multivalent feedback inhibition of aspartokinase in Bacillus polymyxa. I. Effect of nonpolar L-amino acids. J Biol Chem. 1968 Apr 10;243(7):1349–1355. [PubMed] [Google Scholar]
- Paulus H., Gray E. Multivalent feedback inhibition of aspartokinase in Bacillus polymyxa. I. Kinetic studies. J Biol Chem. 1967 Nov 10;242(21):4980–4986. [PubMed] [Google Scholar]
- Rogers A. H. The vitamin requirements of some oral streptococci. Arch Oral Biol. 1973 Feb;18(2):227–232. doi: 10.1016/0003-9969(73)90142-8. [DOI] [PubMed] [Google Scholar]
- Stahly D. P., Bernlohr R. W. Control of aspartokinase during development of Bacillus licheniformis. Biochim Biophys Acta. 1967;146(2):467–476. doi: 10.1016/0005-2744(67)90230-6. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Shockman G. D. Amino acid requirements of Streptococcus mutans and other oral streptococci. Infect Immun. 1975 Apr;11(4):656–664. doi: 10.1128/iai.11.4.656-664.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Regulation of amino acid metabolism. Annu Rev Biochem. 1969;38:323–370. doi: 10.1146/annurev.bi.38.070169.001543. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Critchley P. The extracellular polysaccharide produced from sucrose by a cariogenic streptococcus. Arch Oral Biol. 1966 Oct;11(10):1039–1042. doi: 10.1016/0003-9969(66)90204-4. [DOI] [PubMed] [Google Scholar]