Abstract
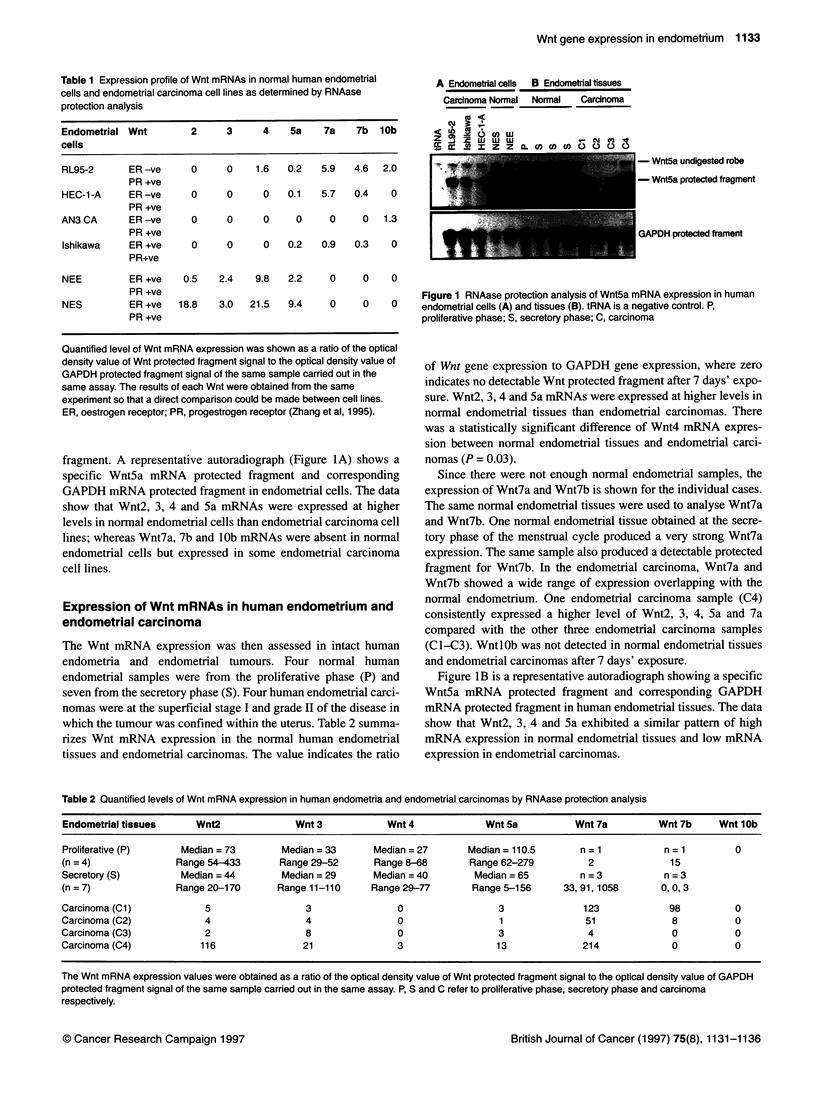
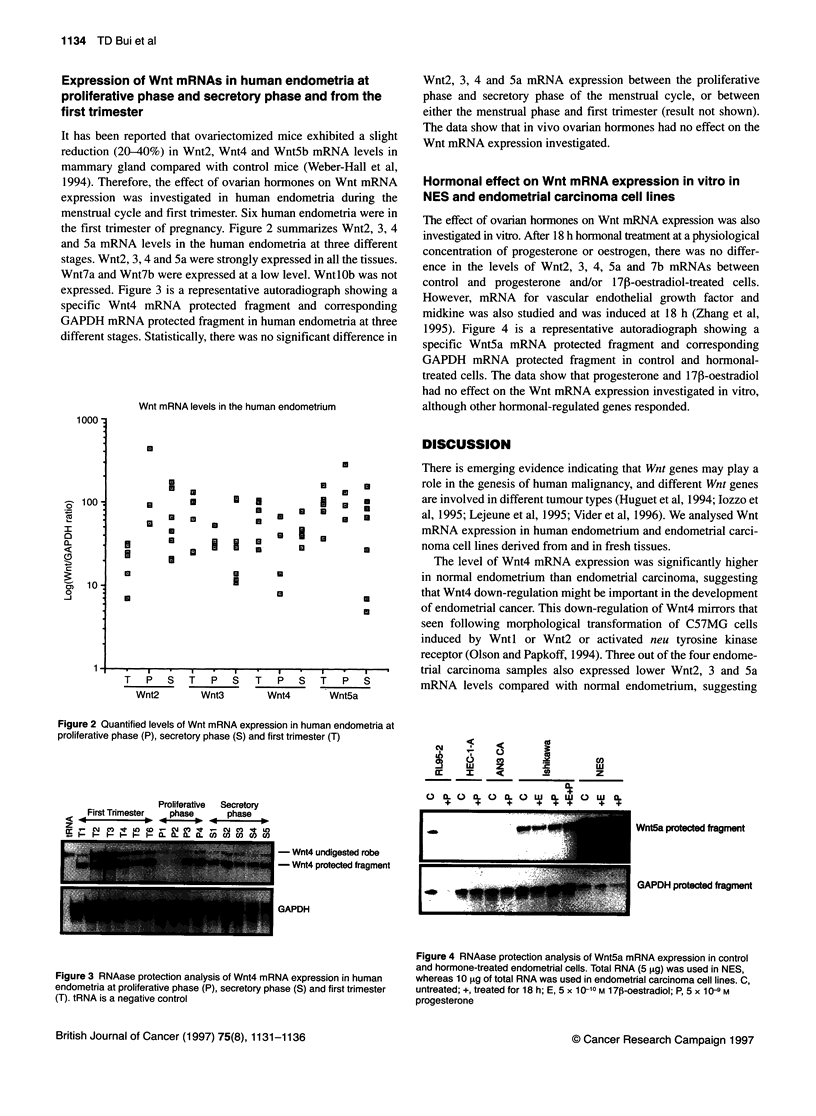
Wnt genes are transforming to mouse breast epithelium and are hormonally regulated in vivo. To assess their role in another endocrine-responsive human cancer, the expression of seven Wnt genes (Wnt 2, 3, 4, 5a, 7a, 7b and 10b) in normal human endometrium and endometrial cells, and endometrial carcinoma tissues and cell lines was investigated by ribonuclease protection analysis. Wnt2, 3, 4 and 5a mRNAs but not Wnt7a, 7b or 10b mRNAs were expressed in primary culture of normal endometrial epithelial (NEE) and stromal (NES) cells. In contrast, in four endometrial carcinoma cell lines (RL95-2, HEC-1-A, AN3 CA and Ishikawa), Wnt2 and Wnt3 mRNAs were absent. Wnt4 was expressed in only one out of four cell lines (RL95-2), and Wnt5a was much lower. Wnt7a and Wnt7b mRNAs were expressed in three out of four cell lines (RL95-2, HEC-1-A and Ishikawa). Wnt10b mRNA was expressed in RL95-2 and AN3 CA. In fresh tissues, all Wnt genes apart from Wnt10b were expressed in normal endometrium and endometrial carcinoma. Similar to the cell lines, the level of Wnt4 mRNA expression was significantly higher in the normal endometrium than endometrial carcinoma. Wnt2, 3 and 5a mRNAs were also lower in endometrial carcinoma compared with normal endometrium. There was no difference in the level of Wnt2, 3, 4 and 5a mRNA expression between proliferative phase and secretory phase of the menstrual cycle, or between either menstrual phase and the first trimester of pregnancy. In vitro, progesterone and/or 17beta-oestradiol had no effect on Wnt2, 3, 4, 5a and 7b mRNA expression in NES and all endometrial carcinoma cell lines. The data indicate that all Wnt genes were expressed in vitro, six out of seven Wnt genes (Wnt 2, 3, 4, 5a, 7a and 7b) were expressed endogenously in the human endometrium, their mRNA expression was hormonally independent and Wnt4 gene down-regulation as well as down-regulation of Wnt 2, 3 and 5a may be associated with endometrial carcinoma.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbury J. M., Niemeyer C. C., Dale T. C., Edwards P. A. Alterations of the growth characteristics of the fibroblast cell line C3H 10T1/2 by members of the Wnt gene family. Oncogene. 1994 Sep;9(9):2597–2603. [PubMed] [Google Scholar]
- Bradley R. S., Cowin P., Brown A. M. Expression of Wnt-1 in PC12 cells results in modulation of plakoglobin and E-cadherin and increased cellular adhesion. J Cell Biol. 1993 Dec;123(6 Pt 2):1857–1865. doi: 10.1083/jcb.123.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler T. A., Dale T. C., Kieback C., Humphreys R. C., Rosen J. M. Localization and quantification of Wnt-2 gene expression in mouse mammary development. Dev Biol. 1993 Jan;155(1):87–96. doi: 10.1006/dbio.1993.1009. [DOI] [PubMed] [Google Scholar]
- Clark C. C., Cohen I., Eichstetter I., Cannizzaro L. A., McPherson J. D., Wasmuth J. J., Iozzo R. V. Molecular cloning of the human proto-oncogene Wnt-5A and mapping of the gene (WNT5A) to chromosome 3p14-p21. Genomics. 1993 Nov;18(2):249–260. doi: 10.1006/geno.1993.1463. [DOI] [PubMed] [Google Scholar]
- Edwards P. A., Hiby S. E., Papkoff J., Bradbury J. M. Hyperplasia of mouse mammary epithelium induced by expression of the Wnt-1 (int-1) oncogene in reconstituted mammary gland. Oncogene. 1992 Oct;7(10):2041–2051. [PubMed] [Google Scholar]
- Gavin B. J., McMahon A. P. Differential regulation of the Wnt gene family during pregnancy and lactation suggests a role in postnatal development of the mammary gland. Mol Cell Biol. 1992 May;12(5):2418–2423. doi: 10.1128/mcb.12.5.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L., Nelson W. J., Papkoff J. Wnt-1 modulates cell-cell adhesion in mammalian cells by stabilizing beta-catenin binding to the cell adhesion protein cadherin. J Cell Biol. 1994 Mar;124(5):729–741. doi: 10.1083/jcb.124.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguet E. L., McMahon J. A., McMahon A. P., Bicknell R., Harris A. L. Differential expression of human Wnt genes 2, 3, 4, and 7B in human breast cell lines and normal and disease states of human breast tissue. Cancer Res. 1994 May 15;54(10):2615–2621. [PubMed] [Google Scholar]
- Huguet E. L., Smith K., Bicknell R., Harris A. L. Regulation of Wnt5a mRNA expression in human mammary epithelial cells by cell shape, confluence, and hepatocyte growth factor. J Biol Chem. 1995 May 26;270(21):12851–12856. doi: 10.1074/jbc.270.21.12851. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V., Eichstetter I., Danielson K. G. Aberrant expression of the growth factor Wnt-5A in human malignancy. Cancer Res. 1995 Aug 15;55(16):3495–3499. [PubMed] [Google Scholar]
- Kwan H., Pecenka V., Tsukamoto A., Parslow T. G., Guzman R., Lin T. P., Muller W. J., Lee F. S., Leder P., Varmus H. E. Transgenes expressing the Wnt-1 and int-2 proto-oncogenes cooperate during mammary carcinogenesis in doubly transgenic mice. Mol Cell Biol. 1992 Jan;12(1):147–154. doi: 10.1128/mcb.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F. S., Lane T. F., Kuo A., Shackleford G. M., Leder P. Insertional mutagenesis identifies a member of the Wnt gene family as a candidate oncogene in the mammary epithelium of int-2/Fgf-3 transgenic mice. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2268–2272. doi: 10.1073/pnas.92.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune S., Huguet E. L., Hamby A., Poulsom R., Harris A. L. Wnt5a cloning, expression, and up-regulation in human primary breast cancers. Clin Cancer Res. 1995 Feb;1(2):215–222. [PubMed] [Google Scholar]
- Lin T. P., Guzman R. C., Osborn R. C., Thordarson G., Nandi S. Role of endocrine, autocrine, and paracrine interactions in the development of mammary hyperplasia in Wnt-1 transgenic mice. Cancer Res. 1992 Aug 15;52(16):4413–4419. [PubMed] [Google Scholar]
- McCarthy S. A., Bicknell R. Responses of pertussis toxin-treated microvascular endothelial cells to transforming growth factor beta 1. No evidence for pertussis-sensitive G-protein involvement in TGF-beta signal transduction. J Biol Chem. 1992 Oct 25;267(30):21617–21622. [PubMed] [Google Scholar]
- Moon R. T., Campbell R. M., Christian J. L., McGrew L. L., Shih J., Fraser S. Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development. 1993 Sep;119(1):97–111. doi: 10.1242/dev.119.1.97. [DOI] [PubMed] [Google Scholar]
- Nusse R., Brown A., Papkoff J., Scambler P., Shackleford G., McMahon A., Moon R., Varmus H. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell. 1991 Jan 25;64(2):231–231. doi: 10.1016/0092-8674(91)90633-a. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Wnt genes. Cell. 1992 Jun 26;69(7):1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- Olson D. J., Papkoff J. Regulated expression of Wnt family members during proliferation of C57mg mammary cells. Cell Growth Differ. 1994 Feb;5(2):197–206. [PubMed] [Google Scholar]
- Pavlova A., Boutin E., Cunha G., Sassoon D. Msx1 (Hox-7.1) in the adult mouse uterus: cellular interactions underlying regulation of expression. Development. 1994 Feb;120(2):335–345. doi: 10.1242/dev.120.2.335. [DOI] [PubMed] [Google Scholar]
- Roelink H., Wagenaar E., Lopes da Silva S., Nusse R. Wnt-3, a gene activated by proviral insertion in mouse mammary tumors, is homologous to int-1/Wnt-1 and is normally expressed in mouse embryos and adult brain. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4519–4523. doi: 10.1073/pnas.87.12.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelink H., Wang J., Black D. M., Solomon E., Nusse R. Molecular cloning and chromosomal localization to 17q21 of the human WNT3 gene. Genomics. 1993 Sep;17(3):790–792. doi: 10.1006/geno.1993.1412. [DOI] [PubMed] [Google Scholar]
- Vider B. Z., Zimber A., Chastre E., Prevot S., Gespach C., Estlein D., Wolloch Y., Tronick S. R., Gazit A., Yaniv A. Evidence for the involvement of the Wnt 2 gene in human colorectal cancer. Oncogene. 1996 Jan 4;12(1):153–158. [PubMed] [Google Scholar]
- Wainwright B. J., Scambler P. J., Stanier P., Watson E. K., Bell G., Wicking C., Estivill X., Courtney M., Boue A., Pedersen P. S. Isolation of a human gene with protein sequence similarity to human and murine int-1 and the Drosophila segment polarity mutant wingless. EMBO J. 1988 Jun;7(6):1743–1748. doi: 10.1002/j.1460-2075.1988.tb03003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Hall S. J., Phippard D. J., Niemeyer C. C., Dale T. C. Developmental and hormonal regulation of Wnt gene expression in the mouse mammary gland. Differentiation. 1994 Sep;57(3):205–214. doi: 10.1046/j.1432-0436.1994.5730205.x. [DOI] [PubMed] [Google Scholar]
- Wong G. T., Gavin B. J., McMahon A. P. Differential transformation of mammary epithelial cells by Wnt genes. Mol Cell Biol. 1994 Sep;14(9):6278–6286. doi: 10.1128/mcb.14.9.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Rees M. C., Bicknell R. The isolation and long-term culture of normal human endometrial epithelium and stroma. Expression of mRNAs for angiogenic polypeptides basally and on oestrogen and progesterone challenges. J Cell Sci. 1995 Jan;108(Pt 1):323–331. doi: 10.1242/jcs.108.1.323. [DOI] [PubMed] [Google Scholar]
- van Ooyen A., Kwee V., Nusse R. The nucleotide sequence of the human int-1 mammary oncogene; evolutionary conservation of coding and non-coding sequences. EMBO J. 1985 Nov;4(11):2905–2909. doi: 10.1002/j.1460-2075.1985.tb04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]






