Abstract
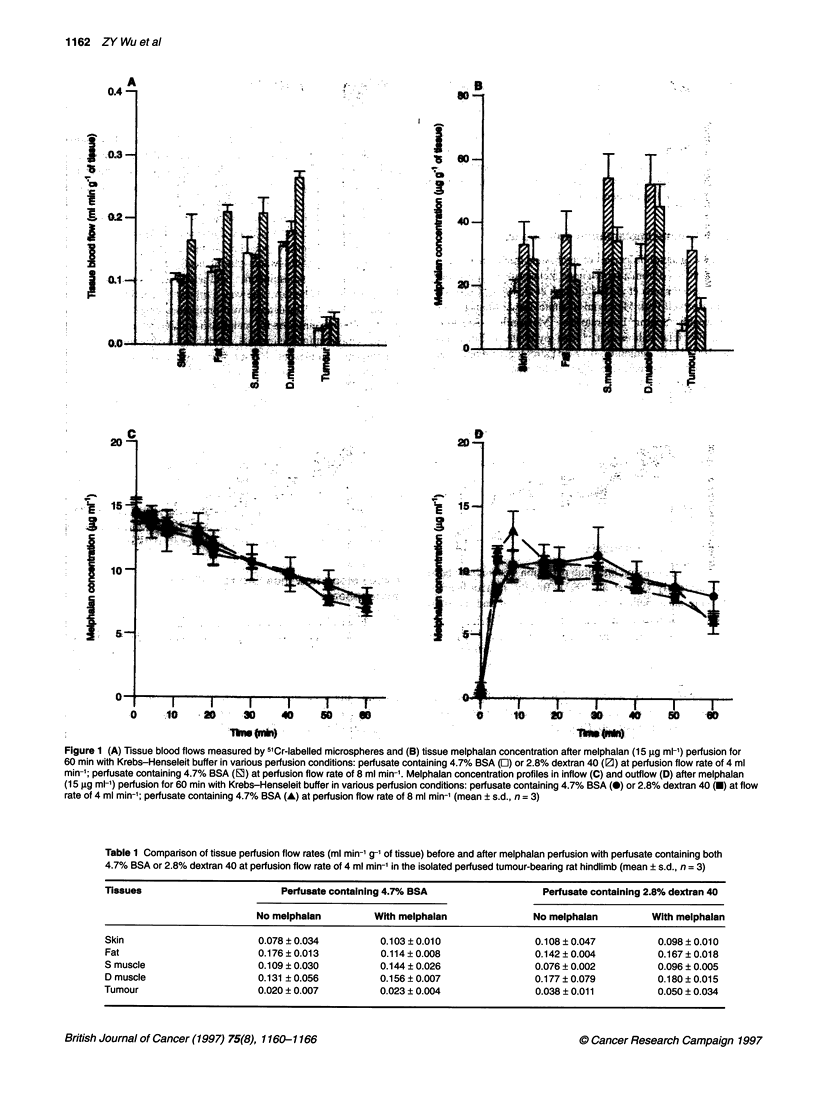
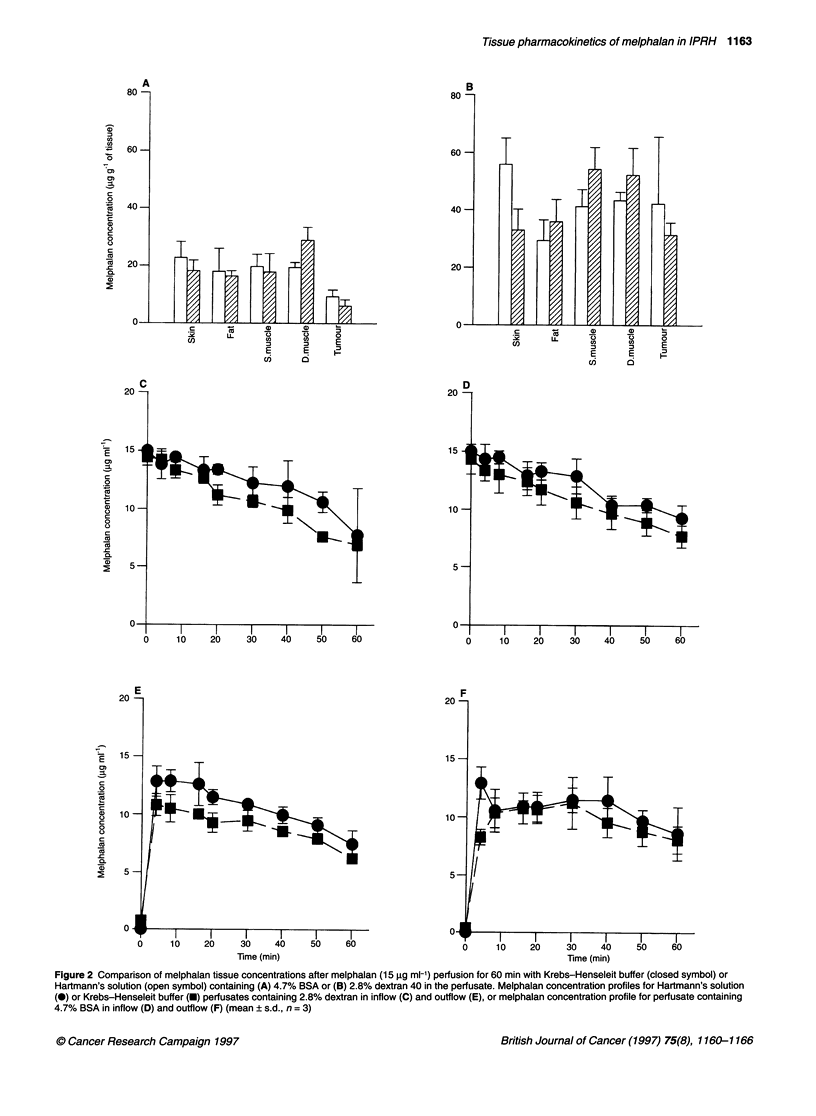
An isolated rat hindlimb perfusion model carrying xenografts of the human melanoma cell line MM96 was used to study the effects of perfusion conditions on melphalan distribution. Krebs-Henseleit buffer and Hartmann's solution containing 4.7% bovine serum albumin (BSA) or 2.8% dextran 40 were used as perfusates. Melphalan concentrations in perfusate, tumour nodules and normal tissues were measured using high-performance liquid chromatography (HPLC). Increasing the perfusion flow rates (from 4 to 8 ml min(-1)) resulted in higher tissue blood flow (determined with 51Cr-labelled microspheres) and melphalan uptake by tumour and normal tissues. The distribution of melphalan within tumour nodules and normal tissues was similar for both Krebs-Henseleit buffer and Hartmann's solution; however, tissue concentrations of melphalan were significantly higher for a perfusate containing 2.8% dextran 40 than for one containing 4.7% BSA. The melphalan concentration in the tumour was one-third of that found in the skin if the perfusate contained 4.7% BSA. In conclusion, this study has shown that a high perfusion flow enhances the delivery of melphalan into implanted tumour nodules and normal tissues, and a perfusate with low melphalan binding (no albumin) is preferred for maximum uptake of drug by the tumour.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benckhuijsen C., Kroon B. B., van Geel A. N., Wieberdink J. Regional perfusion treatment with melphalan for melanoma in a limb: an evaluation of drug kinetics. Eur J Surg Oncol. 1988 Apr;14(2):157–163. [PubMed] [Google Scholar]
- Benckhuijsen C., Varossieau F. J., Hart A. A., Wieberdink J., Noordhoek J. Pharmacokinetics of melphalan in isolated perfusion of the limbs. J Pharmacol Exp Ther. 1986 May;237(2):583–588. [PubMed] [Google Scholar]
- Chang S. Y., Alberts D. S., Farquhar D., Melnick L. R., Walson P. D., Salmon S. E. Hydrolysis and protein binding of melphalan. J Pharm Sci. 1978 May;67(5):682–684. doi: 10.1002/jps.2600670530. [DOI] [PubMed] [Google Scholar]
- Clark J., Grabs A. J., Parsons P. G., Smithers B. M., Addison R. S., Roberts M. S. Melphalan uptake, hyperthermic synergism and drug resistance in a human cell culture model for the isolated limb perfusion of melanoma. Melanoma Res. 1994 Dec;4(6):365–370. doi: 10.1097/00008390-199412000-00004. [DOI] [PubMed] [Google Scholar]
- Cross S. E., Wu Z., Roberts M. S. Effect of perfusion flow rate on the tissue uptake of solutes after dermal application using the rat isolated perfused hindlimb preparation. J Pharm Pharmacol. 1994 Oct;46(10):844–850. doi: 10.1111/j.2042-7158.1994.tb03741.x. [DOI] [PubMed] [Google Scholar]
- Englund N. E., Lindstedt E., Vang J. O. Regional perfusion in treatment of sarcomas of the extremities. Acta Chir Scand. 1971;137(3):243–251. [PubMed] [Google Scholar]
- Fontijne W. P., Mook P. H., Koops H. S., Oldhoff J., Wildevuur C. R. Improved tissue perfusion during pressure regulated hyperthermic regional isolated perfusion. A clinical study. Cancer. 1985 Apr 1;55(7):1455–1461. doi: 10.1002/1097-0142(19850401)55:7<1455::aid-cncr2820550706>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Fontijne W. P., de Vries J., Mook P. H., Elstrodt J. M., Oosterhuis J. W., Schraffordt Koops H., Oldhoff J., Wildevuur C. R. Improved tissue perfusion during pressure-regulated hyperthermic regional isolated perfusion in dogs. J Surg Oncol. 1984 May;26(1):69–76. doi: 10.1002/jso.2930260115. [DOI] [PubMed] [Google Scholar]
- Goss P., Parsons P. G. The effect of hyperthermia and melphalan on survival of human fibroblast strains and melanoma cell lines. Cancer Res. 1977 Jan;37(1):152–156. [PubMed] [Google Scholar]
- Hales J. R., King R. B., Fawcett A. A. Observations on the validity of using "NEN-TRAC" microspheres for measuring organ blood flow. Pflugers Arch. 1979 Apr 30;379(3):295–296. doi: 10.1007/BF00581435. [DOI] [PubMed] [Google Scholar]
- Heymann M. A., Payne B. D., Hoffman J. I., Rudolph A. M. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977 Jul-Aug;20(1):55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- Kettelhack C., Kraus T., Hupp T., Manner M., Schlag P. Hyperthermic limb perfusion for malignant melanoma and soft tissue sarcoma. Eur J Surg Oncol. 1990 Aug;16(4):370–375. [PubMed] [Google Scholar]
- Klaase J. M., Kroon B. B., Beijnen J. H., van Slooten G. W., van Dongen J. A. Melphalan tissue concentrations in patients treated with regional isolated perfusion for melanoma of the lower limb. Br J Cancer. 1994 Jul;70(1):151–153. doi: 10.1038/bjc.1994.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaase J. M., Kroon B. B., van Geel B. N., Eggermont A. M., Franklin H. R., Hart G. A. Patient- and treatment-related factors associated with acute regional toxicity after isolated perfusion for melanoma of the extremities. Am J Surg. 1994 Jun;167(6):618–620. doi: 10.1016/0002-9610(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Krementz E. T., Carter R. D., Sutherland C. M., Muchmore J. H. Chemotherapy by regional perfusion for limb melanoma. Am Surg. 1987 Mar;53(3):133–140. [PubMed] [Google Scholar]
- Kroon B. B. Regional isolation perfusion in melanoma of the limbs; accomplishments, unsolved problems, future. Eur J Surg Oncol. 1988 Apr;14(2):101–110. [PubMed] [Google Scholar]
- Lejeune F. J., Lienard D., Ewalenko P. Hyperthermic isolation perfusion of the limbs with cytostatics after surgical excision of sarcomas. World J Surg. 1988 Jun;12(3):345–348. doi: 10.1007/BF01655668. [DOI] [PubMed] [Google Scholar]
- Morgan D. J., Xu C. L. Effect of perfusate pH on reduction of quinidine capillary permeability by albumin in isolated perfused rat heart. Pharm Res. 1994 Dec;11(12):1820–1824. doi: 10.1023/a:1018988005543. [DOI] [PubMed] [Google Scholar]
- Parsons P. G., Carter F. B., Morrison L., Regius Mary Sister Mechanism of melphalan resistance developed in vitro in human melanoma cells. Cancer Res. 1981 Apr;41(4):1525–1534. [PubMed] [Google Scholar]
- Scott R. N., Blackie R., Kerr D. J., Hughes J., Burnside G., MacKie R. M., Byrne D. S., McKay A. J. Melphalan concentration and distribution in the tissues of tumour-bearing limbs treated by isolated limb perfusion. Eur J Cancer. 1992;28A(11):1811–1813. doi: 10.1016/0959-8049(92)90009-q. [DOI] [PubMed] [Google Scholar]
- Scott R. N., Kerr D. J., Blackie R., Hughes J., Burnside G., MacKie R. M., Byrne D. S., McKay A. J. The pharmacokinetic advantages of isolated limb perfusion with melphalan for malignant melanoma. Br J Cancer. 1992 Jul;66(1):159–166. doi: 10.1038/bjc.1992.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. F., Lai D. T., Ingvar C., Kam P. C. Maximizing efficacy and minimizing toxicity in isolated limb perfusion for melanoma. Melanoma Res. 1994 Mar;4 (Suppl 1):45–50. [PubMed] [Google Scholar]
- Vrouenraets B. C., Klaase J. M., Kroon B. B., van Geel B. N., Eggermont A. M., Franklin H. R. Long-term morbidity after regional isolated perfusion with melphalan for melanoma of the limbs. The influence of acute regional toxic reactions. Arch Surg. 1995 Jan;130(1):43–47. doi: 10.1001/archsurg.1995.01430010045009. [DOI] [PubMed] [Google Scholar]
- Wu Z. Y., Cross S. E., Roberts M. S. Influence of physicochemical parameters and perfusate flow rate on the distribution of solutes in the isolated perfused rat hindlimb determined by the impulse-response technique. J Pharm Sci. 1995 Aug;84(8):1020–1027. doi: 10.1002/jps.2600840820. [DOI] [PubMed] [Google Scholar]
- Wu Z. Y., Rivory L. P., Roberts M. S. Physiological pharmacokinetics of solutes in the isolated perfused rat hindlimb: characterization of the physiology with changing perfusate flow, protein content, and temperature using statistical moment analysis. J Pharmacokinet Biopharm. 1993 Dec;21(6):653–688. doi: 10.1007/BF01113500. [DOI] [PubMed] [Google Scholar]
- Wu Z. Y., Thompson M. J., Roberts M. S., Addison R. S., Cannell G. R., Grabs A. J., Smithers B. M. High-performance liquid chromatographic assay for the measurement of melphalan and its hydrolysis products in perfusate and plasma and melphalan in tissues from human and rat isolated limb perfusions. J Chromatogr B Biomed Appl. 1995 Nov 17;673(2):267–279. doi: 10.1016/0378-4347(95)00277-5. [DOI] [PubMed] [Google Scholar]


