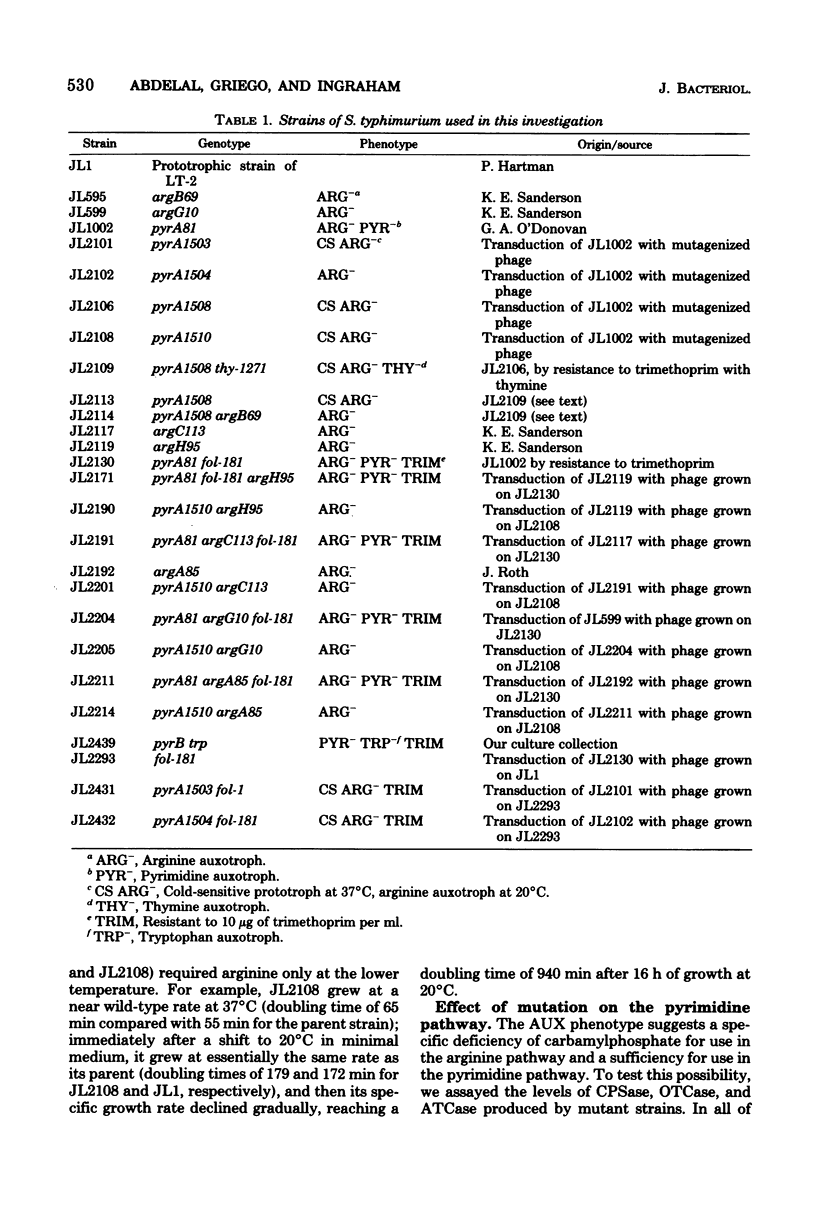
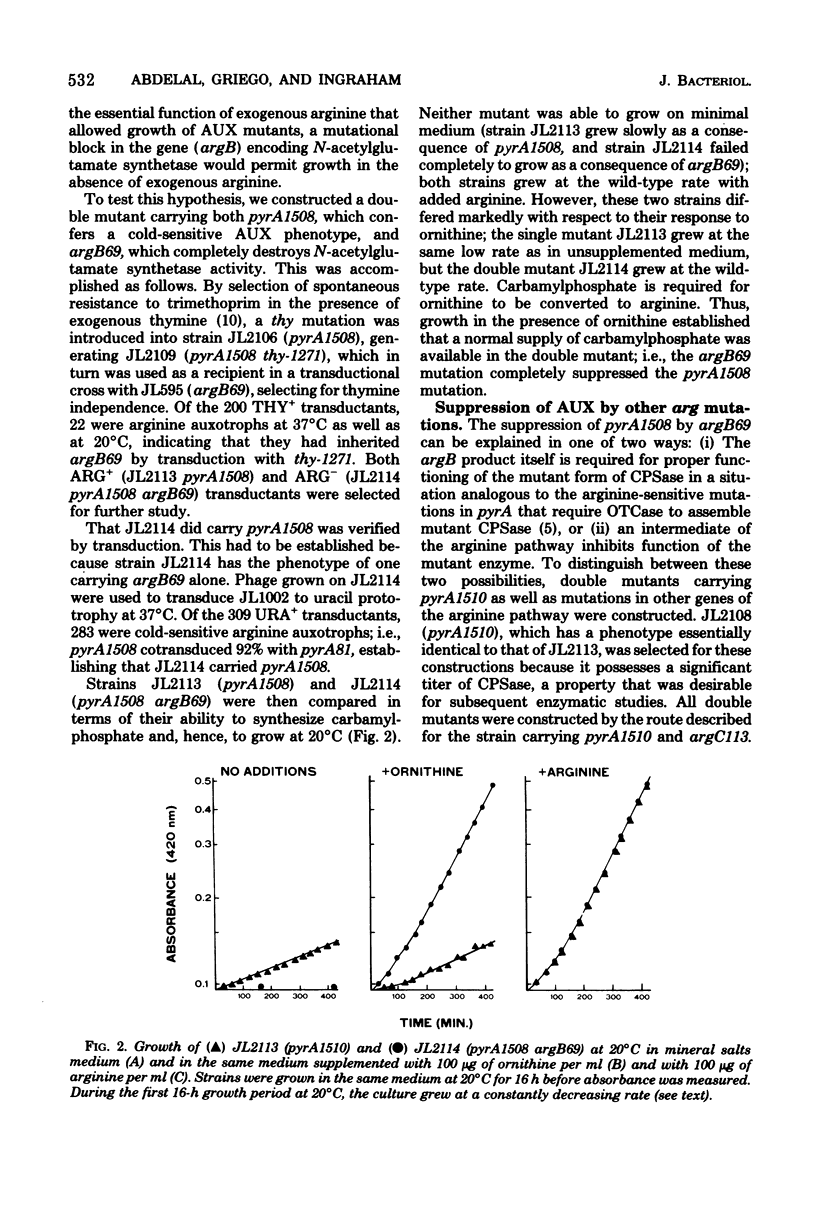
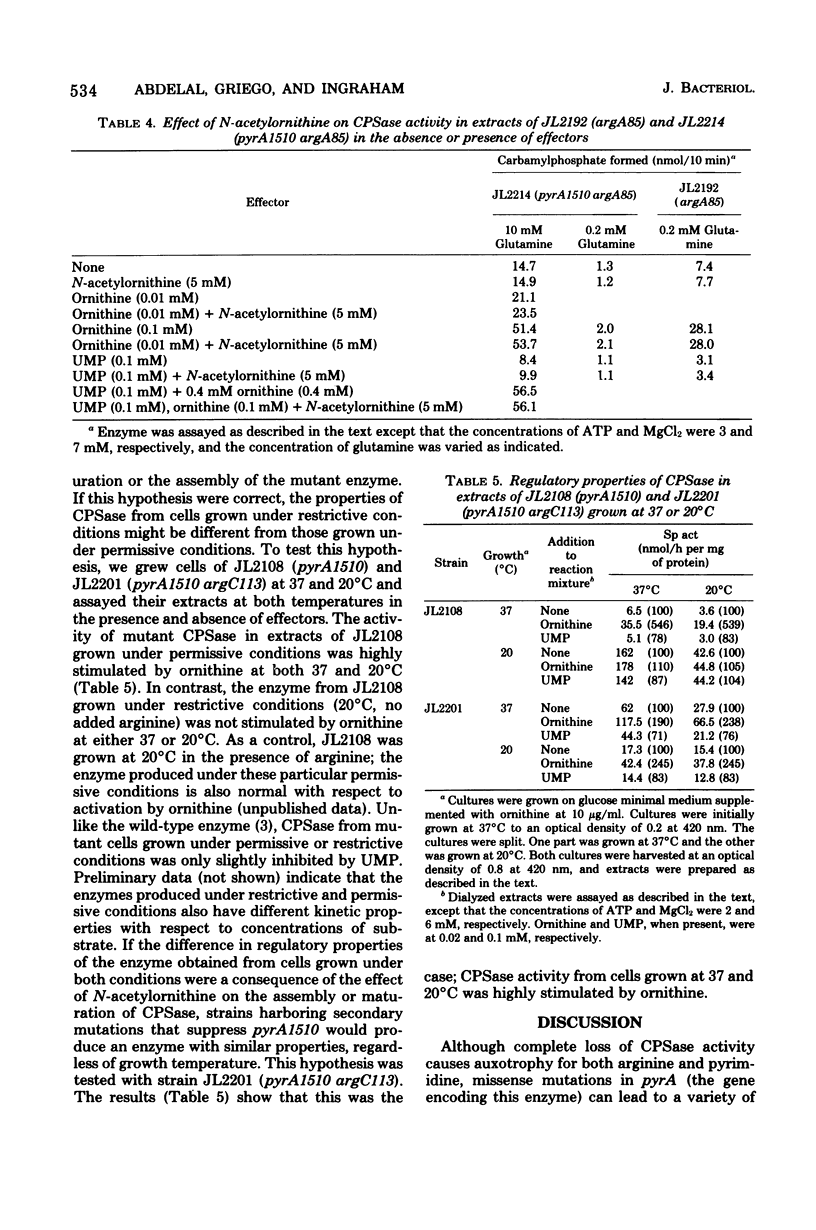
Abstract
Mutations in pyrA that abolish catalytic activity of carbamylphosphate synthetase cause auxotrophy for both arginine and a pyrimidine. Eight pyrA mutants auxotrophic only for arginine (AUX) were isolated by the mutagenized phage technique; three of these required arginine only at low temperature (20°C). Explanations of the AUX phenotype based on bradytrophy were eliminated by the discovery that blocking the utilization of carbamylphosphate for pyrimidine biosynthesis by insertion of an additional mutation in pyrB (encoding aspartic transcarbamylase) did not reduce the requirement for arginine. In contrast, mutational blocks in the arginine biosynthetic pathway before N-acetylornithine (argB, argC, argG, or argH) did suppress the mutation in pyrA. This suggests that exogenous arginine permits growth of the AUX mutants by inhibiting the first step in the arginine pathway, thereby preventing accumulation of an intermediate that antagonizes mutant pyrA function. A mutation in argA (N-acetylornithinase) failed to suppress AUX, indicating that N-acetylornithine was the inhibitory intermediate. This intermediate had no effect on the catalytic or regulatory properties of carbamylphosphate synthetase from mutant cells grown under permissive conditions (37°C). However, the regulatory properties of carbamylphosphate synthetase synthesized under restrictive conditions (20°C) were demonstrably defective (insensitive to activation by ornithine); the enzyme synthesized under permissive conditions was activated by ornithine. A strain carrying an additional mutation (argC), which prevents the accumulation of N-acetylornithine, produced an ornithine-activatable enzyme at both growth temperatures. These results suggest that N-acetylornithine antagonizes the proper preconditioning or maturation of the mutant carbamylphosphate synthetase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A. Arginine-auxotrophic phenotype resulting from a mutation in the pryA gene of Escherichia coli B-r. J Bacteriol. 1969 Jan;97(1):466–468. doi: 10.1128/jb.97.1.466-468.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-el-Al A., Ingraham J. L. Cold sensitivity and other phenotypes resulting from mutation in pyrA gene. J Biol Chem. 1969 Aug 10;244(15):4039–4045. [PubMed] [Google Scholar]
- Abd-el-Al A., Ingraham J. L. Control of carbamyl phosphate synthesis in Salmonella typhimurium. J Biol Chem. 1969 Aug 10;244(15):4033–4038. [PubMed] [Google Scholar]
- Abdelal A. T., Griego E., Ingraham J. L. Arginine-sensitive phenotype of mutations in pyrA of Salmonella typhimurium: role of ornithine carbamyltransferase in the assembly of mutant carbamylphosphate synthetase. J Bacteriol. 1976 Oct;128(1):105–113. doi: 10.1128/jb.128.1.105-113.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelal A. T., Ingraham J. L. Carbamylphosphate synthetase from Salmonella typhimurium. Regulations, subunit composition, and function of the subunits. J Biol Chem. 1975 Jun 25;250(12):4410–4417. [PubMed] [Google Scholar]
- Beck C. F., Ingraham J. L. Location on the chromosome of Salmonella typhimurium of genes governing pyrimidine metabolism. Mol Gen Genet. 1971;111(4):303–316. doi: 10.1007/BF00569782. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Galván M., Martuscelli J. Biochemical and genetic characterization of a carbamyl phosphate synthetase mutant of Escherichia coli K12. J Gen Microbiol. 1976 May;94(1):142–148. doi: 10.1099/00221287-94-1-142. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelln R. A., O'Donovan G. A. Isolation and partial characterization of an argR mutant of Salmonella typhimurium. J Bacteriol. 1976 Nov;128(2):528–535. doi: 10.1128/jb.128.2.528-535.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper J. Gene order and co-transduction in the leu-ara-fol-pyrA region of the Salmonella typhimurium linkage map. J Bacteriol. 1974 Jan;117(1):94–99. doi: 10.1128/jb.117.1.94-99.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marvil D. K., Leisinger T. N-acetylglutamate synthase of Escherichia coli: purification, characterization, and molecular properties. J Biol Chem. 1977 May 25;252(10):3295–3303. [PubMed] [Google Scholar]
- Matthews S. L., Anderson P. M. Evidence for the presence of two nonidentical subunits in carbamyl phosphate synthetase of Escherichia coli. Biochemistry. 1972 Mar 28;11(7):1176–1183. doi: 10.1021/bi00757a010. [DOI] [PubMed] [Google Scholar]
- Mergeay M., Gigot D., Beckmann J., Glansdorff N., Piérard A. Physiology and genetics of carbamoylphosphate synthesis in Escherichia coli K12. Mol Gen Genet. 1974;133(4):299–316. doi: 10.1007/BF00332706. [DOI] [PubMed] [Google Scholar]
- Paulus H., Alpers J. B. Preconditioning: an obligatory step in the biosynthesis of oligomeric enzymes and its promotion by allosteric ligands. Enzyme. 1971;12(4):385–401. doi: 10.1159/000459564. [DOI] [PubMed] [Google Scholar]
- Porter R. W., Modebe M. O., Stark G. R. Aspartate transcarbamylase. Kinetic studies of the catalytic subunit. J Biol Chem. 1969 Apr 10;244(7):1846–1859. [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- Syvanen J. M., Roth J. R. Structural genes for ornithine transcarbamylase in Salmonella typhimurium and Escherichia coli K-12. J Bacteriol. 1972 Apr;110(1):66–70. doi: 10.1128/jb.110.1.66-70.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta P. P., Pinkus L. M., Haschemeyer R. H., Meister A. Reversible dissociation of the monomer of glutamine-dependent carbamyl phosphate synthetase into catalytically active heavy and light subunits. J Biol Chem. 1974 Jan 25;249(2):492–499. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yan Y., Demerec M. Genetic analysis of pyrimidine mutants of Salmonella typhimurium. Genetics. 1965 Sep;52(3):643–651. doi: 10.1093/genetics/52.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]



